BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://jros.iums.ac.ir/article-1-2116-en.html
1. Introduction
McCune-Albright Syndrome (MAS), first described by McCune in 1936 and Albright in 1937 [1], is a sporadic disease characterized by café-au-lait spots (skin involvement), hyperfunctioning endocrinopathies (involvement of certain endocrine organs) and polyostotic fibrous dysplasia (skeleton involvement) [2-8].
Fibrous Dysplasia (FD) is a rare and asymptomatic mosaic disorder caused by replacing a normal bone with fibro-osseous tissues. The new skeleton is weak and fragile and easy to get deformities and fractures [9-14]. FD can affect any part of the body like craniofacial, axial, or appendicular skeleton [15].
Children with FD in appendicular skeleton usually present with limp, pain, and pathological fractures. Pathological fractures usually happen because of the bone weakness due to osteolytic lesions of FD [14].
Using Bisphosphonates (BPs) is one of the ways to reduce pain and frequency of fractures in FD by inhibition of osteoclast-mediated bone resorption [16-20].
Alendronate reduces bone resorption marker but one of the concerns about its long-term use is the atypical femoral fracture [17, 21].
To the best of our knowledge, atypical femoral fracture in MAS has not been reported in the literature [13].
2. Case Presentation
We present a 23-year-old girl (57 kg weight and 155 cm height) with MAS. She had FD lesions, café-au-lait skin spots in the front and back sides of the upper portion of the left side of the chest, and endocrinopathy (Figure 1).
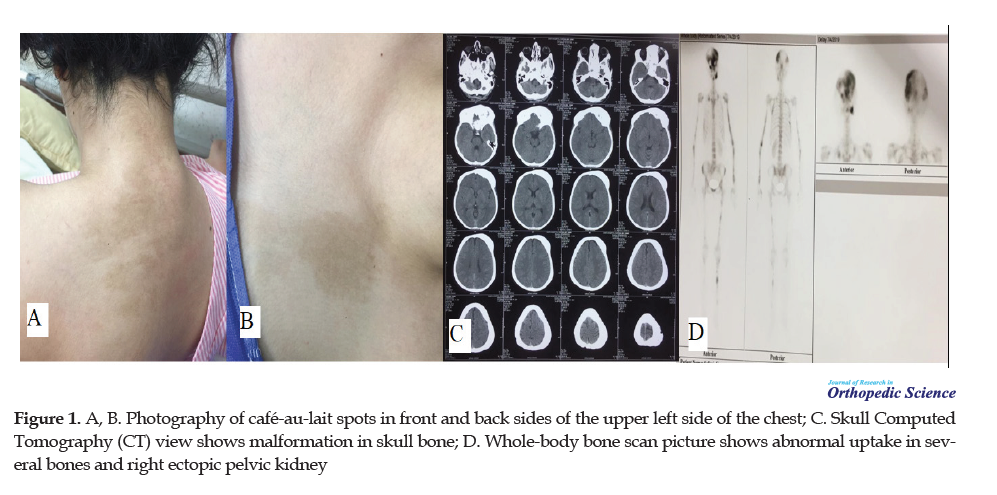
The first time she visited an endocrinologist due to hyperthyroidism was at the age of 15 and then after 4 months, she developed hypothyroidism. Her first menstruation was at the age of 9 years, three years earlier than her older normal sister. There was no familial history of this syndrome. She did not have any other bone complications like osteomalacia. She also had gynecological problems (ovarian cysts) from age 15. She had the right ectopic pelvic kidney without functional impairment (incidental finding in the whole-body bone scan).
She has had walking problems since her young age. When she went to the physician they diagnosed bone cysts but they did not start any medications or use interventions. She also had deformities on the left side of the skull and facial bones.
Her endocrinologist took the whole body bone scan every year to know if the disease improved or not. At the age of 17, she started using alendronate because of mild bone pain and bone cysts. She used alendronate for about 6 years. The intensity of bone pain or the size of the FD lesion had not been changed.
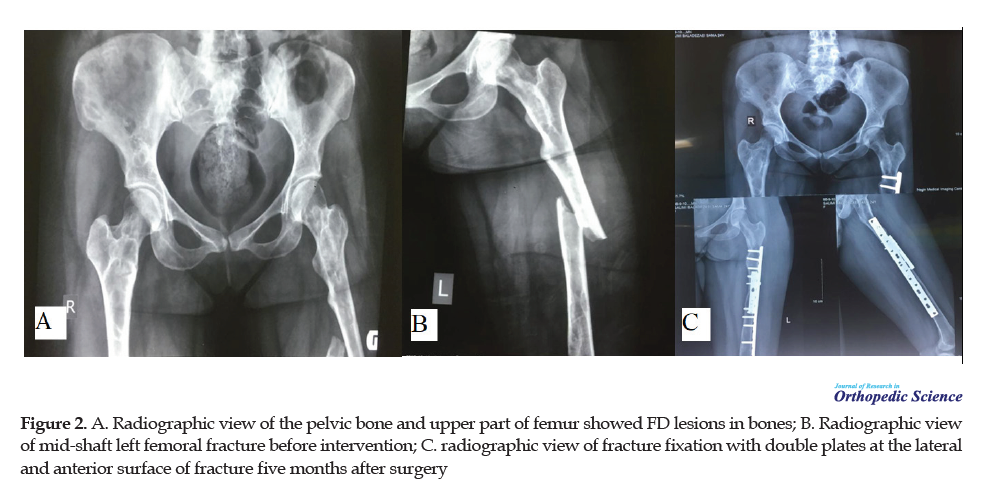
She broke her left femur during simple walking without any trauma 5 months ago. She did not have any prodromal thigh pain before the complete fracture happened. She was admitted to the hospital. Radiographic evaluation showed an atypical mid-shaft fracture in the area that was not involved with FD lesions, proved by microscopic examination of samples taken during surgery. The specimen from the medullary canal of the distal segment of the fracture was fibrous dysplasia lesion. The laboratory tests were normal. By that time her drug history was levothyroxine, metformin, Letroform, and alendronate.
The narrow canal of the femur was not suitable for fixation with an intramedullary interlocking nail. We decided to use double plates with screws for this surgery to increase the strength of fixation and reduce the risk of device failure. Under general anesthesia with a lateral longitudinal incision over the left femur 14-hole hybrid Locking Plate (LCP) were implanted to the lateral aspect of the thigh to fix the fracture. Then, we used a 5-hole Dynamic Compression Plate (DCP) in the anterior surface to double fix the fracture.
Every month we took x-rays to see the way of bone healing progress and rule out the possibility of plate breakage. After the surgery, we banned her from using alendronate. Five months after surgery, a complete :union: was achieved (Figure 2).
3. Discussion and Review of Literature
In this context, we discuss atypical femoral fracture due to the long-term use of alendronate in McCune-Albright syndrome. For this surgery, we used a double plate method containing 14-hole LCP at the lateral aspect of the femur and 5-hole DCP at the anterior surface of the fracture area for double fixation. We did not use intramedullary nailing because of a narrow canal. Alendronate was discontinued. To the best of our knowledge, atypical femoral fracture in MAS was not reported in the literature [13].
McCune-Albright syndrome is a combination of polyostotic FD, café-au-lait spots, and endocrinopathy [2-5, 14, 22, 23]. More than 90% of affected patients are females [2]. Patients with MAS have hyperactivity of endocrine glands that causes precocious puberty, primary hyperthyroidism, and other endocrinopathies [3, 22]. Café-au-lait macules affected 2/3 of patients and usually, they are the earliest presenting features [21].
MAS was first described by McCune in 1936 [24] and Albright in 1937 [25] as an association of FD, skin disorder (café-au-lait spots), and endocrinopathies [1, 14, 26].
FD is a rare disorder caused by replacing the normal bone with fibro-osseous tissue so the new skeleton is weaker and the risk of fracture, deformity, and pain is higher [10, 13, 18, 27]. FD was first described by Lichtenstein in 1938 [28, 29] and can involve any part of the skeleton, like long bones, craniofacial, and axial skeleton. It also has a wide clinical spectrum from affecting one bone (monostotic type) to multiple bones (polyostotic type) or as a part of MAS [18, 28, 29]. FD lesions are detected usually during the first year of life and expand during the next years. [15]. Pain is common in FD but it does not depend on the severity of the disease [28].
Atypical Femoral Fracture (AFF) is a rare condition caused by long-term use of bisphosphonates like alendronate [30]. AFF definition is the fracture located along the femoral diaphysis from just distal to the lesser trochanter to just proximal to the supracondylar flare [31] occurring with minor trauma, has unusual morphology, and has been associated with BPs and other osteoporosis medications [30]. Long-term use of BPs deteriorates the mechanical properties of cortical bones and inhibits bone remodeling mechanism that leads to microdamage in the cortex [31-33].
There have been no reports of atypical femur fractures in MAS despite the high cumulative doses used long-term in some of the reported large case series [13].
Bisphosphonates decrease markers of bone metabolism [11, 32]. Weakly regimen of BPs is one of the options for improvement of bone mineral density in MAS [7]. Despite using alendronate, FD lesions continued to expand and it did not appear to be effective on bone pain [11, 21]. In contrast, Wang et al. studied the efficacy of using BPs like pamidronate and zoledronic acid on bone turn over and pain and it showed improvement of pain and decreases in bone resorption [23]. The long-term use of BPs is defined as more than 3 years of treatment [34]. One of the concerns of long-term use of BPs is impeding bone remodeling and increasing the risk of AFF [13, 17, 31, 32].
According to Lockwood et al., there is a connection between long-term use of BPs and AFF due to microdamage and bone remodeling suppression. There is some evidence suggesting a relationship between the geometry of the lower extremities and the location of AFF. They also reported that patients with long-term use of bisphosphonates should be periodically assessed for thigh pain [31].
The only randomized, double-blind placebo-controlled trial of bisphosphonate use in FD showed that oral alendronate has no effect on bone pain or radiographic appearance compared to placebo [11].
Patients with AFF and history of BPs use are more susceptible to intraoperative and postoperative complications like an iatrogenic fracture, mal:union:, or implant failure than non-BP-related group [16, 31]. Most orthopedic surgeons discontinue any kind of BPs treatment for medical management of AFF due to its effect on bone mineral and suppression of bone remodeling [16]. After the diagnosis of complete or incomplete AFF, using BPs should be stopped [34, 35].
Low energy femur fracture by alendronate was first described in 2005 [35, 36]. We need to use surgical ways like using plates or intramedullary nails to fix these kinds of fractures. People with complete fractures need internal fixation usually with intramedullary nailing [31]. Most surgeons use intramedullary nailing and it is the most common and preferred procedure to fix complete or symptomatic incomplete AFF [16]. Lateral fixation is one of the documented approaches for complete AFF and has good results in patients with incomplete AFF [33].
According to Leet et al., using intramedullary rods could be the most efficient device but using plates and screws prevents eventual loss of fixation [12]. Intramedullary nailing is a basic and the best way for the treatment of complete AFF [35, 37]. Patients with atypical femoral shaft fracture are four times more likely in need of reoperation than those patients with ordinary femoral shaft fracture. One reason for re-surgery is peri-implant fragility fracture that can be prevented by using a cephalomedullary nail [35].
Robinson et al. discussed the surgical management of FD in their article. They said that approaches such as grafting, plates, and screws or external fixations are usually ineffective and should be avoided generally. Intramedullary devices are preferred for lower extremity fractures and deformities [28].
Black et al. reported that there are different techniques for stabilizing fractures and classified them into two groups: extramedullary like plates and screws and intramedullary like intramedullary nails or rods. Bisphosphonates inhibit osteoclastic remodeling so the potential of intramembranous fracture healing decreases and for these kinds of fractures we need extramedullary fixation but most of the orthopedic surgeons recommend using intramedullary nails instead [30].
Ethical Considerations
Compliance with ethical guidelines
Compliance with ethical guidelines: All ethical principles were considered in this article, and the participant and her family were informed about the purpose of the research.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' contributions
Authors contribution's: Conceptualization, design, critical revision of the manuscript for important intellectual content: Salman Ghaffari, Mehran Razavipour; Review of the literature and writing the manuscript: Parastoo Mohammad Amini.
Conflict of interest
The authors declared no conflict of interest.
References
1.Biazzo A, Di Bernardo A, Parafioriti A, Gonfalonieri N. Mazabraud syndrome associated with McCune-Albright syndrome. Acta Biomed. 2017; 88(2):198-200. [DOI:10.23750/abm.v88i2.5256] [PMID] [PMCID]
2.Heller AJ, DiNardo LJ, Massey D. Fibrous dysplasia, chondrosarcoma, and McCune-Albright syndrome. Am J Otolaryngol. 2001; 22(4):297-301. [DOI:10.1053/ajot.2001.24829] [PMID]
3.Hou JW. McCune-Albright syndrome: Diagnosis and clinical course in eleven patients. Pediatr Neonatol. 2018; 59(4):418-20. [DOI:10.1016/j.pedneo.2017.11.005] [PMID]
4.Holbrook L, Brady R. McCune Albright syndrome. Internet: StatPearls Publishing. 2019; [Updated 2019 Jan 20]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK537092/
5.Lietman SA, Ding C, Levine MA. A highly sensitive polymerase chain reaction method detects activating mutations of the GNAS gene in peripheral blood cells in McCune-Albright syndrome or isolated fibrous dysplasia. JBJS. 2005; 87(11):2489-94. [DOI:10.2106/JBJS.E.00160]
6.Boyce A, Turner A, Watts L, Forestier-Zhang L, Underhill A, Pinedo-Villanueva R, et al. Improving patient outcomes in fibrous dysplasia/McCune-Albright syndrome: An international multidisciplinary workshop to inform an international partnership. Arch Osteoporos. 2017; 12(1):21. [DOI:10.1007/s11657-016-0271-6] [PMID] [PMCID]
7.Aragao ALA, Silva IN. Oral alendronate treatment for severe polyostotic fibrous dysplasia due to McCune-Albright syndrome in a child: A case report. Int J Pediatr Endocrinol. 2010; 2010:1-4. [DOI:10.1155/2010/432060] [PMID] [PMCID]
8.Cohen Jr MM. The new bone biology: Pathologic, molecular, and clinical correlates. Am J Med Genet A. 2006; 140(23):2646-706. [DOI:10.1002/ajmg.a.31368] [PMID]
9.Florenzano P, Pan KS, Brown SM, Paul SM, Kushner H, Guthrie LC, et al. Age‐related changes and effects of bisphosphonates on bone turnover and disease progression in fibrous dysplasia of bone. J Bone Miner Res. 2019; 34(4):653-60. [DOI:10.1002/jbmr.3649] [PMID] [PMCID]
10.Hartley I, Zhadina M, Collins MT, Boyce AM. Fibrous dysplasia of bone and McCune-Albright syndrome: A bench to bedside review. Calcif Tissue Int. 2019; 104(5):517-29. [DOI:10.1007/s00223-019-00550-z] [PMID] [PMCID]
11.Boyce AM, Kelly MH, Brillante BA, Kushner H, Wientroub S, Riminucci M, et al. A randomized, double blind, placebo-controlled trial of alendronate treatment for fibrous dysplasia of bone. J Clin Endocrinol Metab. 2014; 99(11):4133-40. [DOI:10.1210/jc.2014-1371] [PMID] [PMCID]
12.Leet AI, Collins MT. Current approach to fibrous dysplasia of bone and McCune-Albright syndrome. J Child Orthop. 2007; 1(1):3-17. [DOI:10.1007/s11832-007-0006-8] [PMID] [PMCID]
13.Majoor BC, Appelman‐Dijkstra NM, Fiocco M, van de Sande MA, Dijkstra PS, Hamdy NA. Outcome of long‐term bisphosphonate therapy in McCune‐Albright syndrome and polyostotic fibrous dysplasia. J Bone Miner Res. 2017; 32(2):264-76. [DOI:10.1002/jbmr.2999] [PMID]
14.Chapurlat RD, Orcel P. Fibrous dysplasia of bone and McCune-Albright syndrome. Best Pract Res Clin Rheumatol. 2008; 22(1):55-69. [DOI:10.1016/j.berh.2007.11.004] [PMID]
15.Burke A, Collins MT, Boyce AM. Fibrous dysplasia of bone: Craniofacial and dental implications. Oral dis. 2017;23(6):697-708. [DOI:10.1111/odi.12563] [PMID] [PMCID]
16.Schneider P, Wall M, Brown J, Cheung A, Harvey E, Morin S. Atypical femur fractures: a survey of current practices in orthopedic surgery. Osteoporos Int. 2017; 28(11):3271-6. [DOI:10.1007/s00198-017-4155-4] [PMID]
17.Simm PJ, Biggin A, Zacharin MR, Rodda CP, Tham E, Siafarikas A, et al. Consensus guidelines on the use of bisphosphonate therapy in children and adolescents. J Paediatr Child Health. 2018; 54(3):223-33. [DOI:10.1111/jpc.13768] [PMID]
18.Kushare IV, Colo D, Bakhshi H, Dormans JP. Fibrous dysplasia of the proximal femur: Surgical management options and outcomes. J Child Orthop. 2014; 8(6):505-11. [DOI:10.1007/s11832-014-0625-9] [PMID] [PMCID]
19.Greenspan S, Vujevich K, Britton C, Herradura A, Gruen G, Tarkin I, et al. Teriparatide for treatment of patients with bisphosphonate-associated atypical fracture of the femur. Osteoporos Int. 2018; 29(2):501-6. [DOI:10.1007/s00198-017-4286-7] [PMID] [PMCID]
20.Corsi A, Ippolito E, Robey PG, Riminucci M, Boyde A. Bisphosphonate-induced zebra lines in fibrous dysplasia of bone: Histo-radiographic correlation in a case of McCune-Albright syndrome. Skeletal radiol. 2017; 46(10):1435-9. [DOI:10.1007/s00256-017-2698-2] [PMID] [PMCID]
21.Boyce AM, Collins MT. Fibrous Dysplasia/McCune-Albright syndrome: A rare, mosaic disease of gα s activation. Endocr Rev. 2020; 41(2):345-70. [DOI:10.1210/endrev/bnz011] [PMID]
22.Völkl TM, Dörr HG. McCune-Albright syndrome: Clinical picture and natural history in children and adolescents. J Pediatr Endocrinol Metab. 2006; 19(Suppl. 2):551-60. [DOI:10.1515/JPEM.2006.19.S2.551] [PMID]
23.Wang Y, Wang O, Jiang Y, Li M, Xia W, Meng X, et al. Efficacy and safety of bisphosphonate therapy in McCune-Albright syndrome-related polyostotic fibrous dysplasia: A single-center experience. Endocr Pract. 2019; 25(1):23-30. [DOI:10.4158/EP-2018-0328] [PMID]
24.McCune D. Osteitis fibrosa cystica: The case of a nine year old girl who also exhibits precocious puberty, multiple pigmentation of the skin and hyperthyroidism. Am J Dis Child. 1936; 52:743-4.
25.Albright F, Butler AM, Hampton AO, Smith P. Syndrome characterized by osteitis fibrosa disseminata, areas of pigmentation and endocrine dysfunction, with precocious puberty in females: Report of five cases. N Engl J Med. 1937; 216(17):727-46. [DOI:10.1056/NEJM193704292161701]
26.Mamkin I, Philibert P, Anhalt H, Ten S, Sultan C. Unusual phenotypical variations in a boy with McCune-Albright syndrome. Horm Res Paediatr. 2010; 73(3):215-22. [DOI:10.1159/000284365] [PMID]
27.Nishida Y, Tsukushi S, Hosono K, Nakashima H, Yamada Y, Urakawa H, et al. Surgical treatment for fibrous dysplasia of femoral neck with mild but prolonged symptoms: A case series. J Orthop Surg Res. 2015; 10(1):1-7. [DOI:10.1186/s13018-015-0208-6] [PMID] [PMCID]
28.Robinson C, Collins MT, Boyce AM. Fibrous dysplasia/McCune-Albright syndrome: Clinical and translational perspectives. Curr Osteoporos Rep. 2016; 14(5):178-86. [DOI:10.1007/s11914-016-0317-0] [PMID] [PMCID]
29.Kaynak BA. Conservative treatment of Fibrous Dysplasia. Pak J Med Sci. 2019; 35(3):873. [DOI:10.12669/pjms.35.3.14] [PMID] [PMCID]
30.Black DM, Abrahamsen B, Bouxsein ML, Einhorn T, Napoli N. Atypical femur fractures: review of epidemiology, relationship to bisphosphonates, prevention, and clinical management. Endocr Rev. 2019; 40(2):333-68. [DOI:10.1210/er.2018-00001] [PMID]
31.Lockwood M, Banderudrappagari R, Suva LJ, Makhoul I. Atypical femoral fractures from bisphosphonate in cancer patients-Review. J Bone Oncol. 2019; 18:100259. [DOI:10.1016/j.jbo.2019.100259] [PMID] [PMCID]
32.Bajaj D, Geissler JR, Allen MR, Burr DB, Fritton JC. The resistance of cortical bone tissue to failure under cyclic loading is reduced with alendronate. Bone. 2014; 64:57-64. [DOI:10.1016/j.bone.2014.03.045] [PMID] [PMCID]
33.Kharazmi M, Michaëlsson K, Hallberg P, Schilcher J. Lateral fixation: An alternative surgical approach in the prevention of complete atypical femoral fractures. Eur J Orthop Surg Traumatol. 2018; 28(2):299-304. [DOI:10.1007/s00590-017-2041-6] [PMID] [PMCID]
34.Khan AA, Kaiser S. Atypical femoral fracture. CMAJ. 2017; 189(14):E542-E. [DOI:10.1503/cmaj.160450] [PMID] [PMCID]
35.Starr J, Tay YKD, Shane E. Current understanding of epidemiology, pathophysiology, and management of atypical femur fractures. Curr Osteoporos Rep. 2018; 16(4):519-29. [DOI:10.1007/s11914-018-0464-6] [PMID] [PMCID]
36.Odvina CV, Zerwekh JE, Rao DS, Maalouf N, Gottschalk FA, Pak CY. Severely suppressed bone turnover: A potential complication of alendronate therapy. J Clin Endocrinol Metab. 2005; 90(3):1294-301. [DOI:10.1210/jc.2004-0952] [PMID]
37.Shane E, Burr D, Abrahamsen B, Adler RA, Brown TD, Cheung AM, et al. Atypical subtrochanteric and diaphyseal femoral fractures: Second report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2014; 29(1):1-23. [DOI:10.1002/jbmr.1998] [PMID]
Received: 2020/05/11 | Accepted: 2020/07/6 | Published: 2020/08/1
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |









