Volume 9, Issue 2 (5-2022)
JROS 2022, 9(2): 111-118 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Hajialiloo Sami S, Sharifi Dalooei S M A, Yaqub Nejad M, Rashidi H, Kargar K. A Huge Distal Radius Giant Cell Tumor: A Case Report. JROS 2022; 9 (2) :111-118
URL: http://jros.iums.ac.ir/article-1-2198-en.html
URL: http://jros.iums.ac.ir/article-1-2198-en.html
Sam Hajialiloo Sami1 

 , Seyyed Mohammad Ata Sharifi Dalooei1
, Seyyed Mohammad Ata Sharifi Dalooei1 

 , Mahdi Yaqub Nejad1
, Mahdi Yaqub Nejad1 
 , Heeva Rashidi1
, Heeva Rashidi1 
 , Khalil Kargar1
, Khalil Kargar1 



 , Seyyed Mohammad Ata Sharifi Dalooei1
, Seyyed Mohammad Ata Sharifi Dalooei1 

 , Mahdi Yaqub Nejad1
, Mahdi Yaqub Nejad1 
 , Heeva Rashidi1
, Heeva Rashidi1 
 , Khalil Kargar1
, Khalil Kargar1 

1- Bone and Joint Reconstruction Research Center, School of Medicine, Shafa Orthopedic Hospital, Iran University of Medical Sciences, Tehran, Iran.
Full-Text [PDF 2856 kb]
(274 Downloads)
| Abstract (HTML) (1358 Views)
Full-Text: (310 Views)
1. Introduction
Giant cell tumor (GCT) of bone is a benign tumor involving the distal end of long bones (meta-epiphyseal) with local invasion and occasional metastasis [1-3], found in ages between 20-40 years [1, 4]. The distal radius is the third common site of involvement, with a higher tendency for recurrence[1, 5].
The treatment goal in distal radius GCT is to completely remove the tumor while preserving maximal wrist function. In this paper, we present a case of huge (12×11×10 cm) distal radius GCT without radiocarpal joint involvement and Campanacci grade 3 [6], treated with wide resection of the tumor and ulnar autograft translocation and wrist arthrodesis using 3.5 mm locking reconstruction plate.
2. Case Presentation
We present a 52-year-old male farm worker who complained of progressive pain, swelling, and limited range of motion of the right wrist for the last 3 years. No history of trauma, body weight loss, or loss of appetite was observed. Laboratory findings, such as erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), leukocyte counts, serum calcium (Ca) concentration, serum phosphorus (P) concentration, serum alkaline phosphatase (Alk-p) were normal.
On physical examination of the right upper limb, an immobile tender huge solid mass exists on the distal right forearm (Figure 1). Due to pain and a huge distal radius mass, the range of motion of the right wrist was limited (0–5° flexion and 0–10° extension). The color and the temperature of the forearm skin were normal [7].
Radiographs showed a purely lytic lesion in the right distal radius extending to the subchondral bone, cortical expansion with cortical thinning, and a narrow zone of transition is proximally observed. It does not cross the joint space without calcification within (Figure 2). A Tc-99 whole-body bone scan revealed increased uptake in the right distal radius and no evidence of further lesions.
On MRI (Figure 3), an expansile solid lesion of the epiphyseal-metaphyseal distal radius with irregular edges that destroys the extensor cortex on the dorsal surface also destroys the cortex on the volar aspect but is contained by the pronator quadratus and narrow zone of transition to normal marrow proximally (Campanacci grade 3 [6]. Distal radius lesion was more consistent with GCT with evidence of local extension into the extensor compartment and some extensor tendons entrapped in the tumor.
Although these images were characteristic of GCT of the distal radius, we performed a core needle biopsy to confirm the diagnosis.
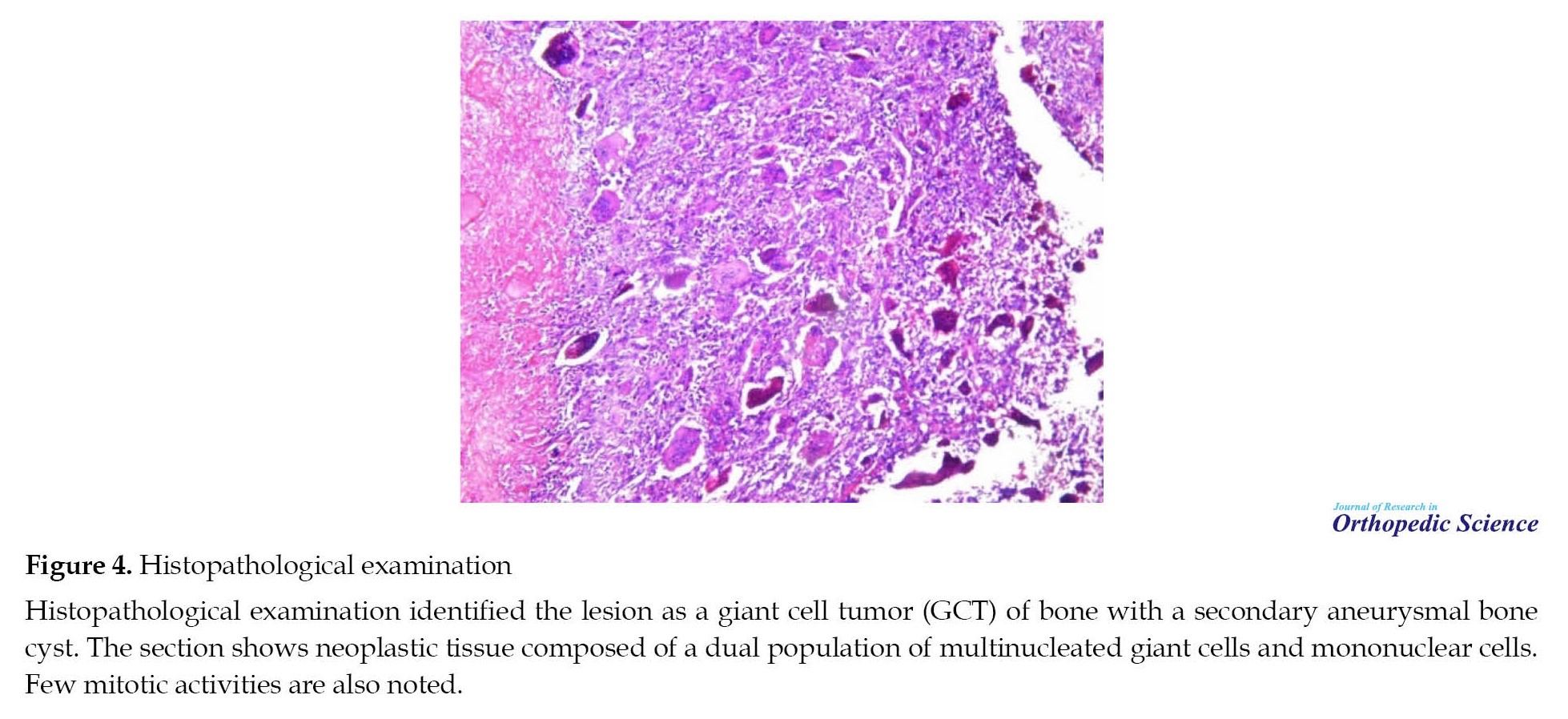
Histopathological examination (Figure 4) identified the lesion as a bone GCT with a secondary aneurysmal bone cyst. Sections show neoplastic tissue composed of a dual population of multinucleated giant cells (typically 40 to 60 nuclei per cell) in a sea of mononuclear stromal cells. Few mitotic activities are also noted. Osteonecrosis and reactive bone necrosis have been observed, as well as clefted space consistent with extensive hemorrhage in the tumor parenchymal has been identified.
We resected the right distal radius and proximal of the proximal row of the carp and then translocate the ipsilateral ulna (with preserving ulnar muscular attachments and osteotomized and excision of the distal end of the ulna) to radius position and wrist arthrodesis with a long 14-hole 3.5 mm reconstruction plate with adding a 4-hole 3.5 mm reconstruction plate at the distal side that overlapped each other because we did not have 16-hole 3.5 mm plate to fix the proximal radius and translocated ulna and up to the third metacarpal bone (Figure 5). We used a double approach (volar and dorsal) to explore and release the tendons and neurovascular bundles, although we cannot preserve abductor pollicis longus (APL), extensor pollicis brevis (EPB), extensor pollicis longus (EPL) tendons at the site of the tumor and we obliged to perform a tendon transfer for these sacrificed tendons. After the surgery, he had a skin problem and wound dehiscence on the radial side of the distal forearm. We taught him how to manipulate and work with his hands to have a good result.
Over the next few months, the symptoms improved, but three months later, the patient was readmitted, complaining of deformity of the right distal forearm (Figure 6). He returned to work after the pain improved and had a device failure proximally. Due to the previous skin condition and stable plate on the distal side, we add an 8-hole 3.5mm dynamic compression plate (DCP) proximal to the previous plate with a cancellous bone graft at the non:union: site (Figure 7). We started physiotherapy two weeks after the first surgery to improve his hand function but he did not cooperate well due to his low socioeconomic status. Therefore, he can flex his interphalangeal joints but cannot flex metacarpal joints more than 60 degrees. Figure 8 shows the favorable condition of the patient’s skin after six months after the first surgery.
3. Discussion
Management of GCT of the distal radius is challenging due to persistent bone destruction and aggressive clinical behavior [8, 9]. Curettage and a bone graft is an acceptable method of treatment for GCT with the good functional outcomes but with high recurrence rate in the distal radius (30-50%) [4, 10]. The treatment of choice for grade 1 and 2 Campanacci tumors is extended curettage and cementing and or bone grafting [9, 11-15]. En-bloc resection is a procedure with a lower recurrence rate, but it creates a challenge in bone reconstruction and is reserved for large Campanacci grade III lesions. Therefore, tumor resection and distal radius reconstruction are preferred in Campanacci grade III cases. This can be achieved by wrist prosthesis, autografts from the tibia, fibula, iliac, allografts, and ulnar translocation [16-20]. Wide resection of the distal radius is recommended for Campanacci grade III GCT when the tumor destructs the dorsal and volar cortex [10].
Reconstruction of the defect of the resected distal radius with fibular non-vascularized autograft has satisfactory functional results, although minor complications occur frequently, such as donor-site problems, leg pain, peroneal nerve injury, and knee instability [6, 15, 19, 21-25]. Compared to vascularized fibular autografts, several authors have reported similar :union: time for non-vascularized fibular autografts (with primary bone grafting and rigid fixation). The most frequent complications are wrist subluxation, delayed :union:, and non-:union:. Superficial infection and soft tissue recurrence are fewer common complications [14, 15, 25, 28].
Several authors stated that the results of fibular autografting were similar to the fibular allograft reconstruction [29, 30, 31]. Allografts are limited in use due to unavailability, increased non-:union:, fracture, infection, probably transmission of diseases, risk of immunological reaction, and requirements of specialized bone bank facilities [14, 29, 32].
Due to the high incidence of carpal subluxation, several authors recommend arthrodesis rather than an arthroplasty [19, 33]. In a study of 67 patients with distal radius GCT who underwent en-bloc excision and reconstruction with osteoarticular grafts or wrist arthrodesis, the results showed no advantages of these two techniques on each other [5].
Distal radius resection with ipsilateral ulna translocation was first described 30 years ago by Seradge who reported good results with painless extremity and acceptable range of motion [34]. This is an easy technique, quicker to perform than free vascularized fibular autografting, facilitates skin closure after tumor excision and the :union: rate is higher compared to non-vascularized grafts [33, 35].
In a study, 14 patients with a Campanacci grade III GCT of the distal radius were treated by en-bloc excision and reconstruction with ulnar translocation with wrist joint arthrodesis. Their results were favorable, and finally this technique was recommended [16]. The use of vascularized ulnar grafts was superior to fibular grafts (even vascularized or nonvascularized) [4]. Translocation of the ulna has often been used with good results but may cause an hourglass appearance in the wrist and distal forearm [16, 33, 36].
Jamshidi et al. perform ulnar translocation with limited wrist arthrodesis in the management of Campanacci grade III GCT of the distal radius. They believe that it preserves the wrist flexion extension to some extent, which is restricted in an arthrodesis [37].
Our patient was classified as grade III according to the Campanacci radiological grading method for giant cell tumors of bone. Radiographs and MRI of the right distal forearm showed an epiphyseal-metaphyseal lesion in one-third of the distal right radius with a narrow transition zone that destroyed the dorsal and volar cortex.
In the case of Campanacci grade III GCT of the distal radius, we performed extensive resection and reconstruction of the bony defect with ulnar translocation and wrist arthrodesis with a 3.5 mm reconstruction plate system. This technique did not need graft and reduced donor site morbidity; in addition, after recovery and transplantation, the patient’s ability to go to physical work is desirable.
In the 18-month follow-up, the patient showed satisfactory functional improvement and good pain relief.
To conclude, we believe that although results show a slight loss of function compared to contra lateral upper extremity, they provide acceptable results to our patient with a huge distal radius GCT. This technique reduces the time of surgery and also does not require specialized bone bank facilities (for fibular allograft) or microvascular surgery (for fibular vascularized autograft reconstruction).
Ethical Considerations
Compliance with ethical guidelines
All ethical principles were considered in this article. The participants were informed of the purpose of the research and its implementation stages. They were also assured about the confidentiality of their information and were free to leave the study whenever they wished, and if desired, the research results would be available to them.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors equally contributed to preparing this article.
Conflict of interest
The authors declared no conflict of interest.
References
Giant cell tumor (GCT) of bone is a benign tumor involving the distal end of long bones (meta-epiphyseal) with local invasion and occasional metastasis [1-3], found in ages between 20-40 years [1, 4]. The distal radius is the third common site of involvement, with a higher tendency for recurrence[1, 5].
The treatment goal in distal radius GCT is to completely remove the tumor while preserving maximal wrist function. In this paper, we present a case of huge (12×11×10 cm) distal radius GCT without radiocarpal joint involvement and Campanacci grade 3 [6], treated with wide resection of the tumor and ulnar autograft translocation and wrist arthrodesis using 3.5 mm locking reconstruction plate.
2. Case Presentation
We present a 52-year-old male farm worker who complained of progressive pain, swelling, and limited range of motion of the right wrist for the last 3 years. No history of trauma, body weight loss, or loss of appetite was observed. Laboratory findings, such as erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), leukocyte counts, serum calcium (Ca) concentration, serum phosphorus (P) concentration, serum alkaline phosphatase (Alk-p) were normal.
On physical examination of the right upper limb, an immobile tender huge solid mass exists on the distal right forearm (Figure 1). Due to pain and a huge distal radius mass, the range of motion of the right wrist was limited (0–5° flexion and 0–10° extension). The color and the temperature of the forearm skin were normal [7].
Radiographs showed a purely lytic lesion in the right distal radius extending to the subchondral bone, cortical expansion with cortical thinning, and a narrow zone of transition is proximally observed. It does not cross the joint space without calcification within (Figure 2). A Tc-99 whole-body bone scan revealed increased uptake in the right distal radius and no evidence of further lesions.
On MRI (Figure 3), an expansile solid lesion of the epiphyseal-metaphyseal distal radius with irregular edges that destroys the extensor cortex on the dorsal surface also destroys the cortex on the volar aspect but is contained by the pronator quadratus and narrow zone of transition to normal marrow proximally (Campanacci grade 3 [6]. Distal radius lesion was more consistent with GCT with evidence of local extension into the extensor compartment and some extensor tendons entrapped in the tumor.
Although these images were characteristic of GCT of the distal radius, we performed a core needle biopsy to confirm the diagnosis.
Histopathological examination (Figure 4) identified the lesion as a bone GCT with a secondary aneurysmal bone cyst. Sections show neoplastic tissue composed of a dual population of multinucleated giant cells (typically 40 to 60 nuclei per cell) in a sea of mononuclear stromal cells. Few mitotic activities are also noted. Osteonecrosis and reactive bone necrosis have been observed, as well as clefted space consistent with extensive hemorrhage in the tumor parenchymal has been identified.
We resected the right distal radius and proximal of the proximal row of the carp and then translocate the ipsilateral ulna (with preserving ulnar muscular attachments and osteotomized and excision of the distal end of the ulna) to radius position and wrist arthrodesis with a long 14-hole 3.5 mm reconstruction plate with adding a 4-hole 3.5 mm reconstruction plate at the distal side that overlapped each other because we did not have 16-hole 3.5 mm plate to fix the proximal radius and translocated ulna and up to the third metacarpal bone (Figure 5). We used a double approach (volar and dorsal) to explore and release the tendons and neurovascular bundles, although we cannot preserve abductor pollicis longus (APL), extensor pollicis brevis (EPB), extensor pollicis longus (EPL) tendons at the site of the tumor and we obliged to perform a tendon transfer for these sacrificed tendons. After the surgery, he had a skin problem and wound dehiscence on the radial side of the distal forearm. We taught him how to manipulate and work with his hands to have a good result.
Over the next few months, the symptoms improved, but three months later, the patient was readmitted, complaining of deformity of the right distal forearm (Figure 6). He returned to work after the pain improved and had a device failure proximally. Due to the previous skin condition and stable plate on the distal side, we add an 8-hole 3.5mm dynamic compression plate (DCP) proximal to the previous plate with a cancellous bone graft at the non:union: site (Figure 7). We started physiotherapy two weeks after the first surgery to improve his hand function but he did not cooperate well due to his low socioeconomic status. Therefore, he can flex his interphalangeal joints but cannot flex metacarpal joints more than 60 degrees. Figure 8 shows the favorable condition of the patient’s skin after six months after the first surgery.
3. Discussion
Management of GCT of the distal radius is challenging due to persistent bone destruction and aggressive clinical behavior [8, 9]. Curettage and a bone graft is an acceptable method of treatment for GCT with the good functional outcomes but with high recurrence rate in the distal radius (30-50%) [4, 10]. The treatment of choice for grade 1 and 2 Campanacci tumors is extended curettage and cementing and or bone grafting [9, 11-15]. En-bloc resection is a procedure with a lower recurrence rate, but it creates a challenge in bone reconstruction and is reserved for large Campanacci grade III lesions. Therefore, tumor resection and distal radius reconstruction are preferred in Campanacci grade III cases. This can be achieved by wrist prosthesis, autografts from the tibia, fibula, iliac, allografts, and ulnar translocation [16-20]. Wide resection of the distal radius is recommended for Campanacci grade III GCT when the tumor destructs the dorsal and volar cortex [10].
Reconstruction of the defect of the resected distal radius with fibular non-vascularized autograft has satisfactory functional results, although minor complications occur frequently, such as donor-site problems, leg pain, peroneal nerve injury, and knee instability [6, 15, 19, 21-25]. Compared to vascularized fibular autografts, several authors have reported similar :union: time for non-vascularized fibular autografts (with primary bone grafting and rigid fixation). The most frequent complications are wrist subluxation, delayed :union:, and non-:union:. Superficial infection and soft tissue recurrence are fewer common complications [14, 15, 25, 28].
Several authors stated that the results of fibular autografting were similar to the fibular allograft reconstruction [29, 30, 31]. Allografts are limited in use due to unavailability, increased non-:union:, fracture, infection, probably transmission of diseases, risk of immunological reaction, and requirements of specialized bone bank facilities [14, 29, 32].
Due to the high incidence of carpal subluxation, several authors recommend arthrodesis rather than an arthroplasty [19, 33]. In a study of 67 patients with distal radius GCT who underwent en-bloc excision and reconstruction with osteoarticular grafts or wrist arthrodesis, the results showed no advantages of these two techniques on each other [5].
Distal radius resection with ipsilateral ulna translocation was first described 30 years ago by Seradge who reported good results with painless extremity and acceptable range of motion [34]. This is an easy technique, quicker to perform than free vascularized fibular autografting, facilitates skin closure after tumor excision and the :union: rate is higher compared to non-vascularized grafts [33, 35].
In a study, 14 patients with a Campanacci grade III GCT of the distal radius were treated by en-bloc excision and reconstruction with ulnar translocation with wrist joint arthrodesis. Their results were favorable, and finally this technique was recommended [16]. The use of vascularized ulnar grafts was superior to fibular grafts (even vascularized or nonvascularized) [4]. Translocation of the ulna has often been used with good results but may cause an hourglass appearance in the wrist and distal forearm [16, 33, 36].
Jamshidi et al. perform ulnar translocation with limited wrist arthrodesis in the management of Campanacci grade III GCT of the distal radius. They believe that it preserves the wrist flexion extension to some extent, which is restricted in an arthrodesis [37].
Our patient was classified as grade III according to the Campanacci radiological grading method for giant cell tumors of bone. Radiographs and MRI of the right distal forearm showed an epiphyseal-metaphyseal lesion in one-third of the distal right radius with a narrow transition zone that destroyed the dorsal and volar cortex.
In the case of Campanacci grade III GCT of the distal radius, we performed extensive resection and reconstruction of the bony defect with ulnar translocation and wrist arthrodesis with a 3.5 mm reconstruction plate system. This technique did not need graft and reduced donor site morbidity; in addition, after recovery and transplantation, the patient’s ability to go to physical work is desirable.
In the 18-month follow-up, the patient showed satisfactory functional improvement and good pain relief.
To conclude, we believe that although results show a slight loss of function compared to contra lateral upper extremity, they provide acceptable results to our patient with a huge distal radius GCT. This technique reduces the time of surgery and also does not require specialized bone bank facilities (for fibular allograft) or microvascular surgery (for fibular vascularized autograft reconstruction).
Ethical Considerations
Compliance with ethical guidelines
All ethical principles were considered in this article. The participants were informed of the purpose of the research and its implementation stages. They were also assured about the confidentiality of their information and were free to leave the study whenever they wished, and if desired, the research results would be available to them.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors equally contributed to preparing this article.
Conflict of interest
The authors declared no conflict of interest.
References
- Zou C, Lin T, Wang B, Zhao Z, Li B, Xie X, et al. Managements of giant cell tumor within the distal radius: A retrospective study of 58 cases from a single center. J Bone Oncol. 2019; 14:100211. [DOI:10.1016/j.jbo.2018.100211] [PMID] [PMCID]
- Errani C, Ruggieri P, Asenzio MA, Toscano A, Colangeli S, Rimondi E, et al. Giant cell tumor of the extremity: A review of 349 cases from a single institution. Cancer Treat Rev. 2010; 36(1):1-7. [DOI:10.1016/j.ctrv.2009.09.002] [PMID]
- Panchwagh Y, Puri A, Agarwal M, Anchan C, Shah M. Giant cell tumor-distal end radius: Do we know the answer?. Indian J Orthop. 2007; 41(2):139-45. [DOI:10.4103/0019-5413.32046] [PMID] [PMCID]
- Chobpenthai T, Thanindratarn P, Phorkhar T, Ingviya T. The reconstruction after en-bloc resection of giant cell tumors at the distal radius: A systematic review and meta-analysis of the ulnar transposition reconstruction technique. Surg Oncol. 2020; 34:147-53. [DOI:10.1016/j.suronc.2020.04.015] [PMID]
- Bianchi G, Sambri A, Marini E, Piana R, Campanacci DA, Donati DM. Wrist arthrodesis and osteoarticular reconstruction in giant cell tumor of the distal radius. J Hand Surg Am. 2020; 45(9):882.e1-6. [DOI:10.1016/j.jhsa.2020.03.005] [PMID]
- Campanacci M. Giant-cell tumor and chondrosarcomas: Grading, treatment and results (studies of 209 and 131 cases). IRecent Results Cancer Res. 1976; (54):257-61. [DOI:10.1007/978-3-642-80997-2_22] [PMID]
- Jafari D, Najd Mazhar F, Jalili A, Zare S, Hoseini Teshnizi S. The inter and intraobserver reliability of measurements of the distal radius radiographic indices. J Res Orthop Sci. 2014; 1(2):22-5. [Link]
- Szendröi M. Giant-cell tumour of bone. J Bone Joint Surg. Br. 2004; 86(1):5-12. [DOI:10.1302/0301-620X.86B1.14053]
- O’Donnell RJ, Springfield DS, Motwani HK, Ready JE, Gebhardt MC, Mankin HJ. Recurrence of giant-cell tumors of the long bones after curettage and packing with cement. J Bone Joint Surg Am. 1994; 76(12):1827-33. [DOI:10.2106/00004623-199412000-00009] [PMID]
- Saikia KC, Borgohain M, Bhuyan SK, Goswami S, Bora A, Ahmed F. Resection-reconstruction arthroplasty for giant cell tumor of distal radius.Indian J Orthop. 2010; 44(3):327-32. [DOI:10.4103/0019-5413.65134] [PMID] [PMCID]
- Goldenberg RR, Campbell CJ, Bonfiglio M. Giant-cell tumor of bone: An analysis of two hundred and eighteen cases. J Bone Joint Surg . 1970; 52(4):619-64. [DOI:10.2106/00004623-197052040-00001]
- Lackman RD, McDonald DJ, Beckenbaugh RD, Sim FH. Fibular reconstruction for giant cell tumor of the distal radius. Clin Orthop Relat Res. 1987; (218):232-8. [DOI:10.1097/00003086-198705000-00032]
- Mcdonald DJ, Sim FH, McLeod RA, Dahlin DC. Giant-cell tumor of bone. J Bone Joint Surg. 1986; 68(2):235-42. [DOI:10.2106/00004623-198668020-00009]
- Saini R, Bali K, Bachhal V, Mootha AK, Dhillon MS, Gill SS. En bloc excision and autogenous fibular reconstruction for aggressive giant cell tumor of distal radius: A report of 12 cases and review of literature. J Orthop Surg Res. 2011; 6:14. [DOI:10.1186/1749-799X-6-14] [PMID] [PMCID]
- Chadha M, Arora SS, Singh AP, Gulati D, Singh AP. Autogenous non-vascularized fibula for treatment of giant cell tumor of distal end radius. Arch Orthop Trauma Surg. 2010; 130(12):1467-73. [DOI:10.1007/s00402-010-1059-6] [PMID]
- Puri A, Gulia A, Agarwal MG, Reddy K. Ulnar translocation after excision of a Campanacci grade-3 giant-cell tumour of the distal radius: An effective method of reconstruction. J Bone Joint Surg Br. 2010; 92(6):875-9. [DOI:10.1302/0301-620X.92B6.23194] [PMID]
- Campbell CJ, Akbarnia BA. Giant-cell tumor of the radius treated by massive resection and tibial bone graft. J Bone and Joint surg. 1975; 57(7):982-6. [DOI:10.2106/00004623-197557070-00018]
- Leung PC, Chan KT. Giant cell tumor of the distal end of the radius treated by the resection and free vascularized iliac crest graft. Clin Orthop Relat Res. 1986; 202:232-6. [DOI:10.1097/00003086-198601000-00033]
- Murray JA, Schlafly B. Giant-cell tumors in the distal end of the radius. Treatment by resection and fibular autograft interpositional arthrodesis. J Bone Joint Surg. 1986; 68(5):687-94. [DOI:10.2106/00004623-198668050-00008]
- Turcotte RE, Wunder JS, Isler MH, Bell RS, Schachar N, Masri BA, et al. Giant cell tumor of long bone: A Canadian Sarcoma Group study. Clin Orthop Relat Res. 2002; 397:248-58. [DOI:10.1097/00003086-200204000-00029] [PMID]
- Salenius P, Santavirta S, Kiviluoto O, Koskinen EV. Application of free autogenous fibular graft in the treatment of aggressive bone tumours of the distal end of the radius. Arch Orthop Trauma Surg (1978). 1981; 98(4):285-7. [DOI:10.1007/BF00378882] [PMID]
- Maruthainar N, Zambakidis C, Harper G, Calder D, Cannon SR, Briggs TW. Functional outcome following excision of tumours of the distal radius and reconstruction by autologous non-vascularized osteoarticular fibula grafting. J Hand Surg Br. 2002; 27(2):171-4. [DOI:10.1054/JHSB.2001.0707] [PMID]
- Aithal VK, Bhaskaranand K. Reconstruction of the distal radius by fibula following excision of giant cell tumor. Int Orthop. 2003; 27(2):110-3. [DOI:10.1007/s00264-002-0414-9] [PMID] [PMCID]
- Harris WR, Lehmann EC. Recurrent giant-cell tumour after en bloc excision of the distal radius and fibular autograft replacement. J Bone Joint Surg Br. 1983; 65(5):618-20. [DOI:10.1302/0301-620X.65B5.6643568] [PMID]
- Jafari D, Shariatzadeh H, Okhovatpour MA, Razavipour M, Safdari F. Giant cell tumor of distal radius: En bloc resection and partial wrist arthrodesis using non-vascularized fibular autograft. J Res Orthop Sci. 2017; 4(2):e11774. [DOI:10.5812/soj.11774]
- Pho RW. Malignant giant-cell tumor of the distal end of the radius treated by a free vascularized fibular transplant. The Journal of Bone and Joint surgery. 1981; 63(6):877-84. [DOI:10.2106/00004623-198163060-00003]
- Hsu R. Free vascularised fibular grafting for reconstruction after tumour resection. J Bone Joint Surg Br. 1997; 79(1):36-42. [DOI:10.1302/0301-620X.79B1.0790036]
- Pho RW. Free vascularised fibular transplant for replacement of the lower radius. J Bone Joint Surg Br. 1979; 61-B(3):362-5. [DOI:10.1302/0301-620X.61B3.479261] [PMID]
- Kocher MS, Gebhardt MC, Mankin HJ. Reconstruction of the distal aspect of the radius with use of an osteoarticular allograft after excision of a skeletal tumor.J Bone Joint Surg Am. 1998; 80(3):407-19. [DOI:10.2106/00004623-199803000-00014] [PMID]
- Szabo RM, Anderson KA, Chen JL. Functional outcome of en bloc excision and osteoarticular allograft replacement with the Sauve-Kapandji procedure for Campanacci grade 3 giant-cell tumor of the distal radius. J Hand Surg Am. 2006; 31(8):1340-8. [DOI:10.1016/j.jhsa.2006.06.004] [PMID]
- Bianchi G, Donati D, Staals EL, Mercuri M. Osteoarticular allograft reconstruction of the distal radius after bone tumour resection. J Hand Surg Br. 2005; 30(4):369-70. [DOI:10.1016/J.JHSB.2005.04.006] [PMID]
- Asavamongkolkul A, Waikakul S, Phimolsarnti R, Kiatisevi P. Functional outcome following excision of a tumour and reconstruction of the distal radius. Int Orthop. 2009; 33(1):203-9. [DOI:10.1007/s00264-007-0441-7] [PMID] [PMCID]
- Vander Griend RA, Funderburk CH. The treatment of giant-cell tumors of the distal part of the radius. J Bone Joint Surg Am. 1993; 75(6):899-908. [DOI:10.2106/00004623-199306000-00011] [PMID]
- Seradge H. Distal ulnar translocation in the treatment of giant-cell tumors of the distal end of the radius. J Bone Joint surg. 1982; 64(1):67-73. [DOI:10.2106/00004623-198264010-00011]
- Hackbarth DA Jr. Resections and reconstructions for tumors of the distal radius. Orthop Clin North Am. 1991; 22(1):49-64. [DOI:10.1016/S0030-5898(20)31631-X] [PMID]
- Bhan S, Biyani A. Ulnar translocation after excision of giant cell tumour of distal radius. J Hand Surg. 1990; 15(4):496-500. [DOI:10.1016/0266-7681(90)90102-A]
- Jamshidi K, Heidari M, Mirzaei A. Ulnar translocation with limited arthrodesis in the management of giant cell tumours of the distal radius. J Hand Surg Eur Vol. 2020; 45(4):420-1. [DOI:10.1177/1753193419899830] [PMID]
Type of Study: Case Report |
Subject:
Tumor surgery
Received: 2022/06/5 | Accepted: 2022/10/29 | Published: 2022/05/1
Received: 2022/06/5 | Accepted: 2022/10/29 | Published: 2022/05/1
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |