Volume 9, Issue 4 (11-2022)
JROS 2022, 9(4): 209-218 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Ebrahimzadeh Babaki A, Ebrahimzadeh Babaki F, Mortazavizadeh M R. Survival Analysis of Osteosarcoma in a University Hospital in Yazd, Iran From 2004 to 2018: A Period Analysis. JROS 2022; 9 (4) :209-218
URL: http://jros.iums.ac.ir/article-1-2220-en.html
URL: http://jros.iums.ac.ir/article-1-2220-en.html
1- Department of Orthopedic, School of Medicine, Iran University of Medical Sciences, Tehran, Iran.
2- Department of Hematology-Oncology, Ali-ebn-Abitaleb Faculty of Medicine, Yazd Branch, Islamic Azad University, Yazd, Iran.
2- Department of Hematology-Oncology, Ali-ebn-Abitaleb Faculty of Medicine, Yazd Branch, Islamic Azad University, Yazd, Iran.
Full-Text [PDF 1055 kb]
(111 Downloads)
| Abstract (HTML) (505 Views)
Full-Text: (169 Views)
1. Introduction
Osteosarcoma (OS) is a malignant bone neoplasm originating from bone mesenchymal cells. It is the most common cause of limb loss due to cancers [1, 2]. It often affects children, teenagers, and young adults. OS has a bimodal distribution, most frequently affecting the youth and the elderly [2-4]. The incidence rate is estimated to be 3.4 per million people annually, and men are affected more than women [5]. This tumor originates in long bones, such as the distal femur, tibia, or humerus [3]. Approximately 15% to 20% of patients have metastases at the time of diagnosis. The lungs and bones are the most common sites of metastasis [2].
Before 1970, chemotherapy was not used for OS, and the survival rate was poor. When surgery was the only treatment method, most patients died one year after diagnosis, and the total 5-year survival rate was around 10% [6, 7]. The standard of care for OS is surgery with neoadjuvant and or adjuvant chemotherapy [6, 8, 9]. The four chemotherapy agents that are included in almost all treatment regimens include methotrexate, doxorubicin, cisplatin, and ifosfamide. Patients with metastasis may also be treated with etoposide [2, 10-13]. Tumor necrosis response to neoadjuvant chemotherapy is the overall response to treatment. Patients with tumor necrosis of more than 90% have a 5-year survival rate of 82%, while subjects with tumor necrosis of less than 90% have a 5-year survival rate of 68% [14]. The dose intensity is also important. In patients with localized OS, waiting more than 21 days after surgery to restart chemotherapy has a significantly high mortality rate. It is recommended to continue chemotherapy in the first 21 days after the operation to maintain the intensity of the dose [15].
Considering the lack of complete and definitive information regarding the choice of treatment based on the survival rate after treatment, this study examines the survival rate in patients with OS. Accordingly, we determine the mean survival time in patients with OS and associated factors.
2. Methods
This retrospective study was conducted on 29 patients with pathology-confirmed OS. Out of all the patients who were referred to the clinic, the number of patients to whom we had access and were able to check all their conditions was 29 patients. All patients who presented to the University Hospital (Shah Vali Hospital) in Yazd, Iran, from April 2004 to December 2018 were included in the study. The patients who could not be contacted were excluded from the study. Written informed consent was obtained from all patients before they participated in the study. Information on patient-related factors (age, sex), and tumor-related factors (recurrence, recurrence site, metastasis) were extracted from the hospital records, and information about living status was collected through phone calls and clinical visits.
We wanted to have an age division in this study to better evaluate adults and children. The age range of our patients was 8 to 42 years. In the fifth edition of the World Health Organization (WHO) classification, bone tumors are the most widely used pathologic classification system [16]. Bone cancers, such as osteosarcoma under the age of 20, are considered children’s cancers. Also, in some well-known articles and book chapters in this field [17, 18], the age group under 20 has been considered a separate group. Therefore, we divided our patients into two groups. Group 1 included the participants under the age of 20 years as children and the second group involved subjects over the age of 20 as adults.
The treatment process for most patients with osteosarcoma that has not metastasized was as follows: First, three courses of neoadjuvant chemotherapy were performed for the patient. Then, surgery was performed. After surgery, three courses of adjuvant chemotherapy were performed. For patients with metastasis, chemotherapy was performed, then surgery was performed if it could be done.
The chemotherapy regimen is cisplatin 70 mg + doxorubicin (adriamycin) 50 mg daily for three consecutive days every three weeks.
Surgery included resection with wide margins (removal of the tumor with a cuff of normal tissue covering it all around). This means the removal of 2 cm of normal tissue or a good anatomical barrier (e.g. fascial layer/articular cartilage) and osteotomy of bone 3-5 cm away from the level of involvement. In cases where large segmental defects were created following resection, reconstruction with a tumoral prosthesis was done. Meanwhile, in cases where the tumor could not be resected or the involvement on the surrounding tissues was aggressive or had caused neurovascular involvement, amputation was performed.
Data analysis
This study used the SPSS software, version 17 to analyze the data. The Kaplan-Meier test was used for survival analysis and the log-rank test was exploited to investigate the effect of risk factors, such as age, sex, local recurrence, and metastasis on patient survival.
3. Results
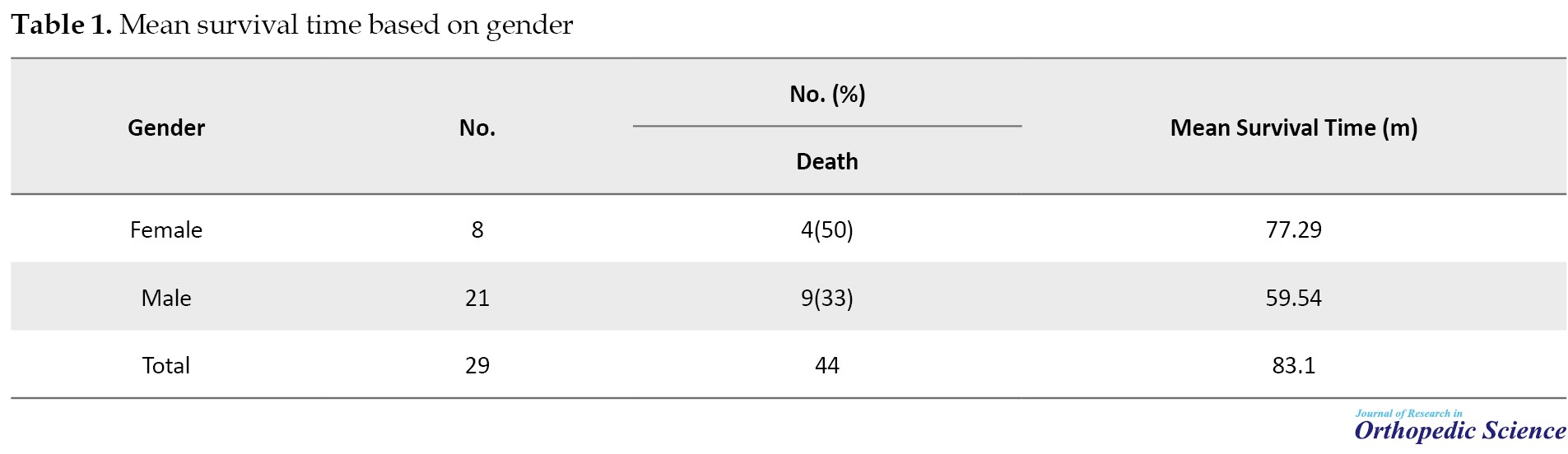
Overall, 29 patients were included in this study, of which 21(72.4%) were male (Table 1).
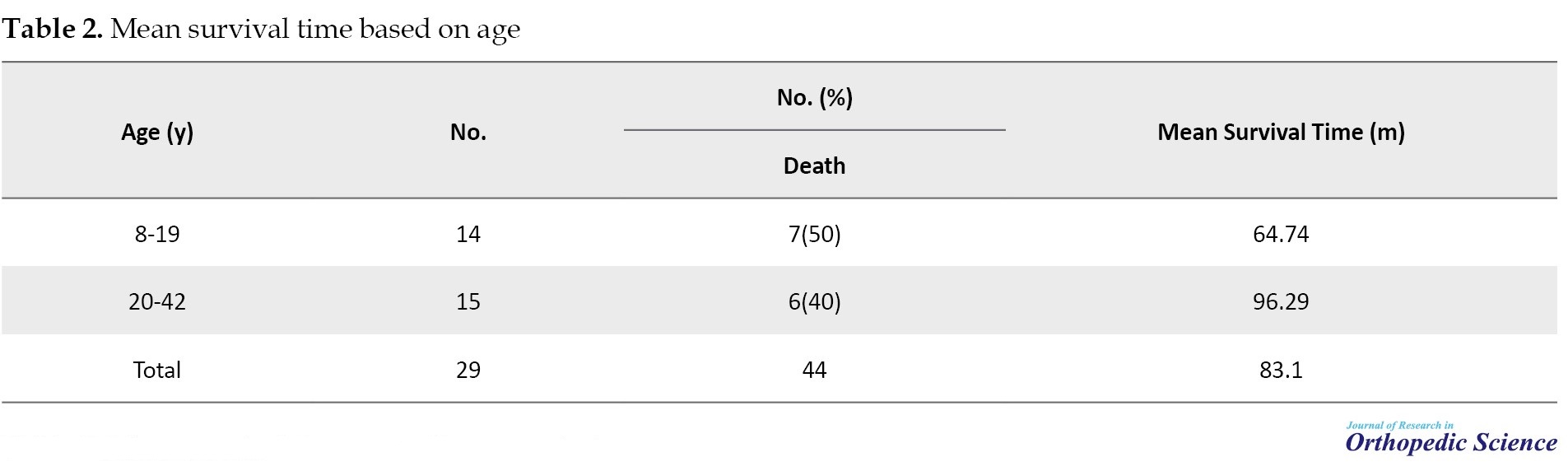
The mean age of patients was 21.3±8.4 (8-42) years (Table 2).
Meanwhile, 4 patients had tumors in the upper limb (all of them in the humerus), 23 patients in the lower limb (2 patients in the pelvis, 12 patients in the femur, and 9 patients in the tibia), and 2 patients had tumors in other areas (mandible and spine).
Thirteen (44.8%) patients died due to OS, and 1(6.9%) patient died due to other reasons (Table 3).
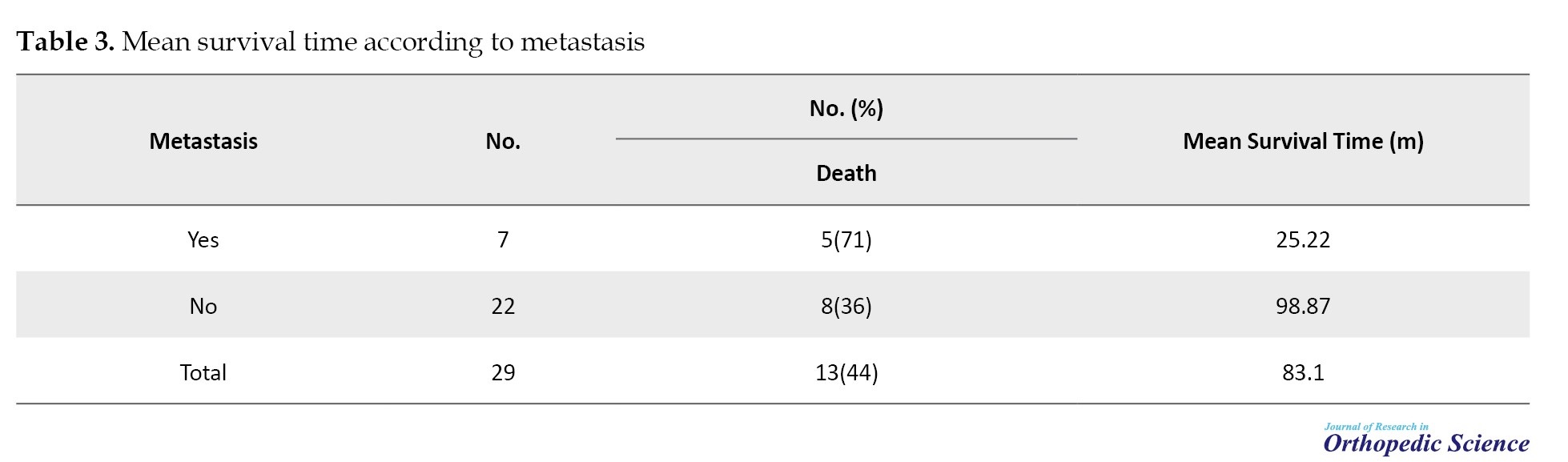
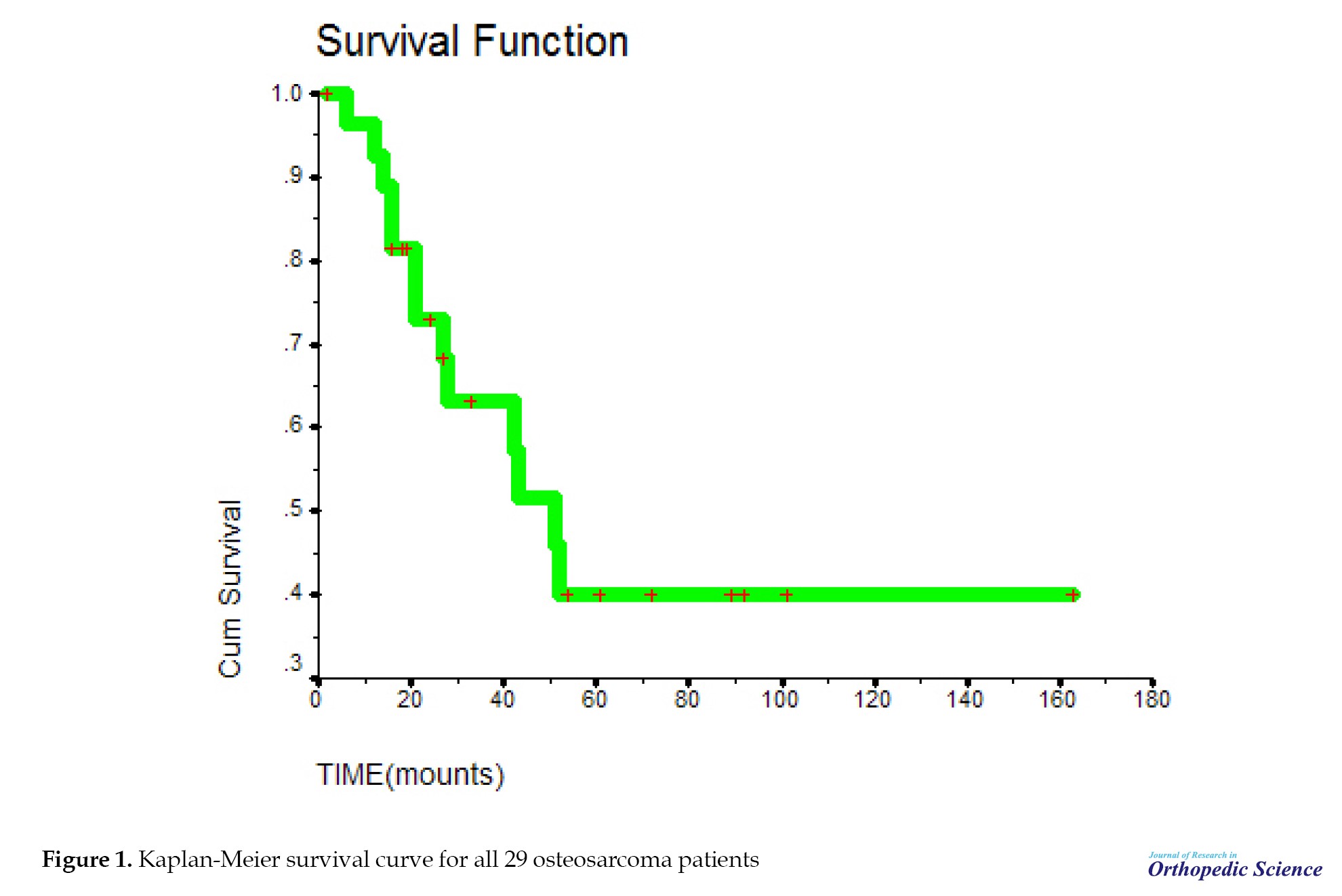
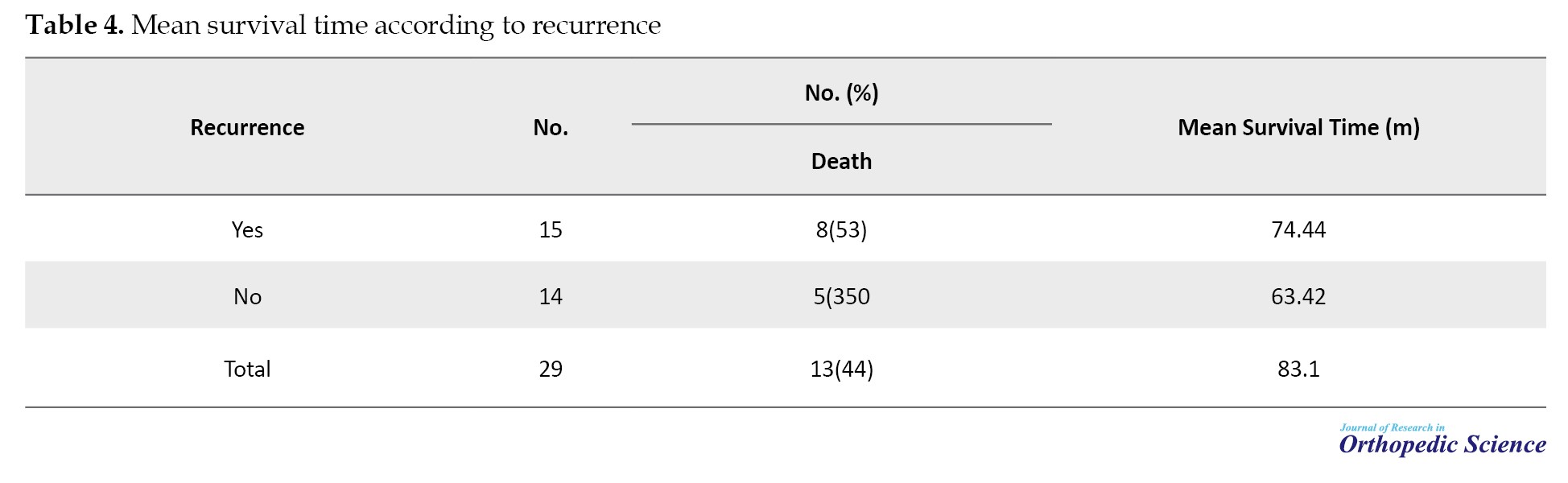
Regarding the type of treatment, 26(89.7%) patients underwent surgery, 26(89.7%) patients had adjuvant chemotherapy, and 27(93.1%) patients had neoadjuvant chemotherapy. The mean survival time in the subjects was 83.1±14.71 (95% CI, 54.27%, 111.92%) months. The median survival time was 51 months. The survival rate of patients for 1-year, 3-year, and 5-year periods was 90%, 64%, and 40%, respectively (Table 4).
The mean tumor necrosis rate was 79.53±28.84 (5%-100%). Meanwhile, 10 cases had a necrosis rate higher than 90%, and 5 patients had a tumor necrosis rate less than 90% (P=0.002). The necrosis rate was higher in the survivors.
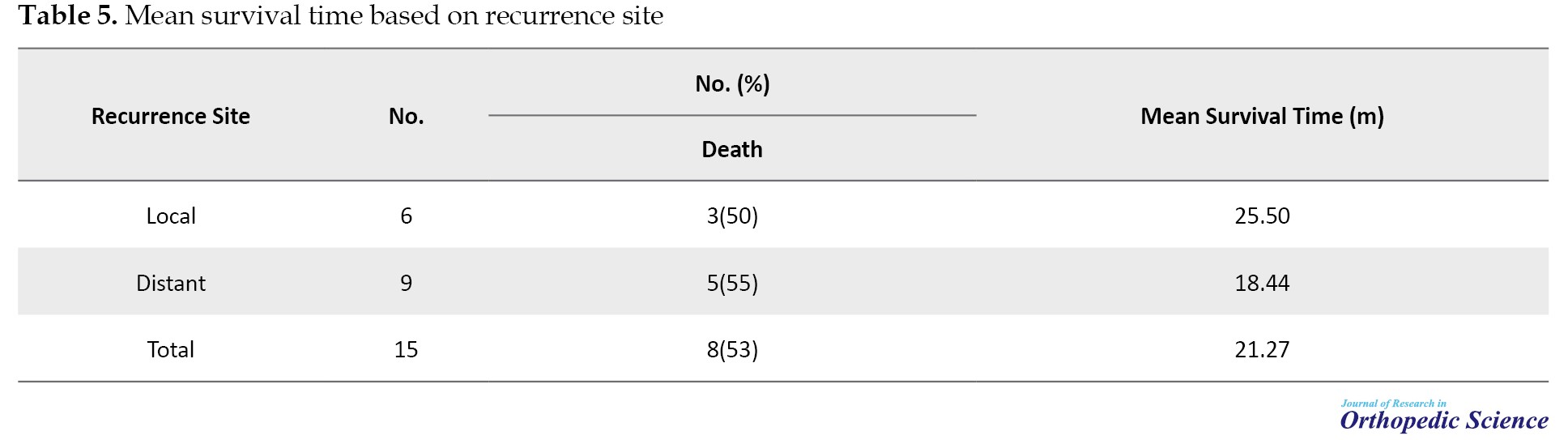
The mean duration of disease-free survival was 38 months, and the patients’ mean survival time after relapse was 21.27±5.07 (95% CI, 11.33%, 31.21%) (Table 5).
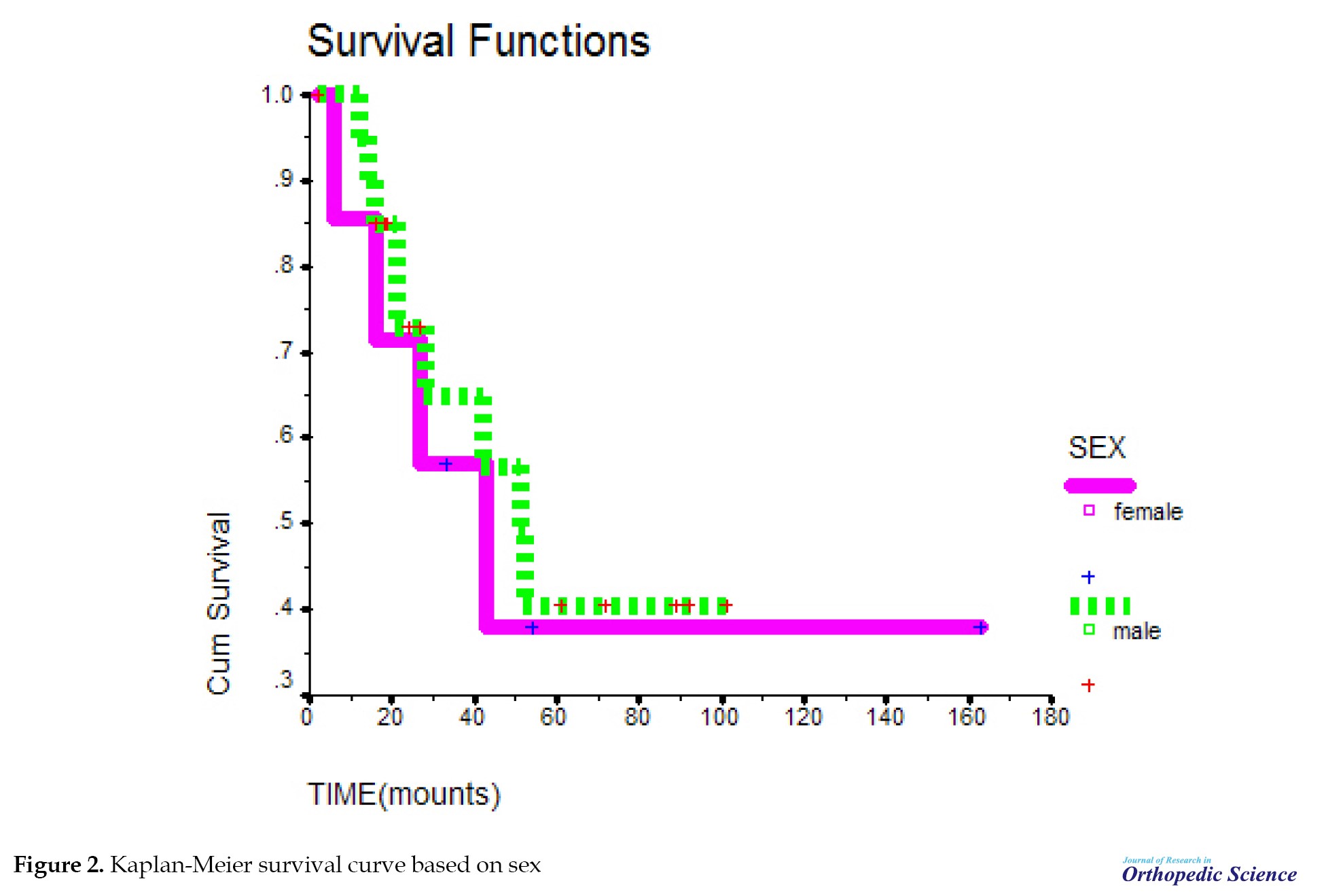
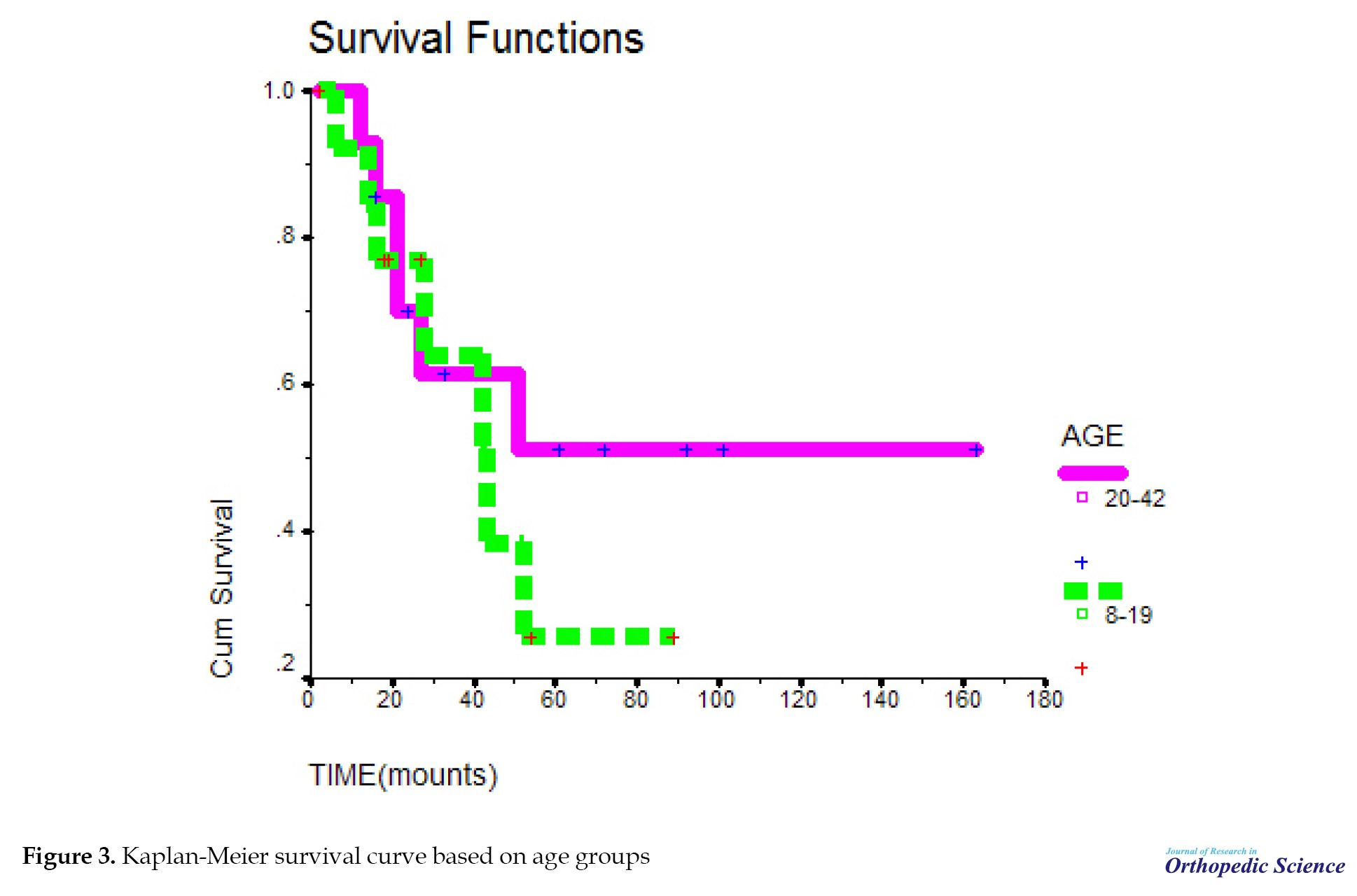
The probability of survival decreased with a relatively sharp slope until 51 months, but from 51 months onward, the probability of survival remained almost constant and did not change. The mean survival time in the studied samples was 83.1. In addition, 1-year survival of patients was about 90%, 3-year survival was about 64%, and 5-year survival was 40% in the studied samples (Figure 1). The probability of survival was the same in both sexes. Survival time in this study was not related to gender (P=0.713) (Figure 2). The mean survival period at the ages of 8-19 years was not significant, compared to the ages of 20-42 years with P=0.4643 (Figure 3). The probability of survival up to 41 months was the same at different ages. After 41 months, the probability of survival remained constant at the ages of 20-42 years; however, at the ages of 8-19 years, the probability of survival decreased, but with the difference of P=0.46, which is not significant.
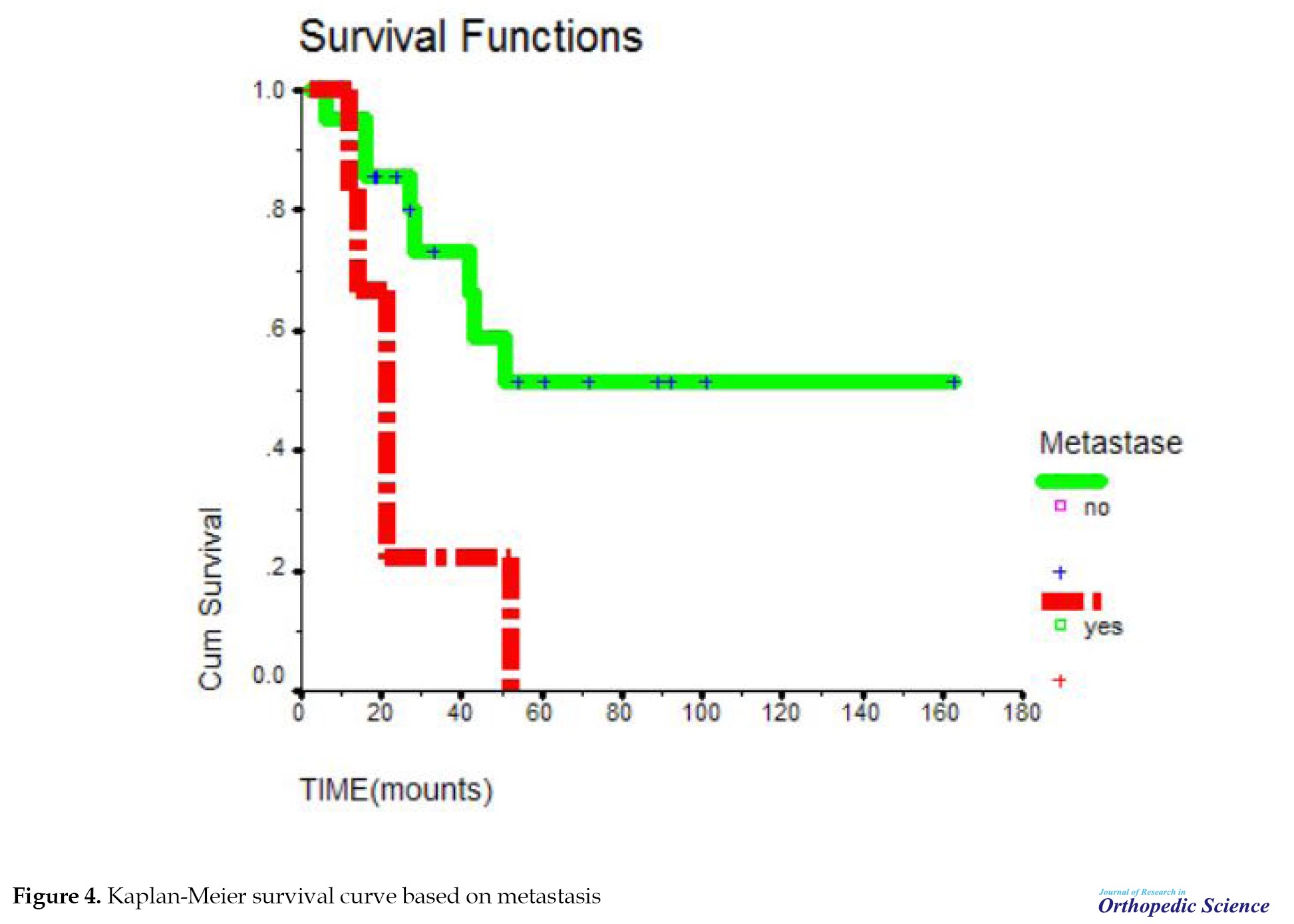
The mean survival time in people who had primary metastasis compared to people without primary metastasis was significant with P=0.0116; accordingly, primary metastasis causes a decrease in survival time (Figure 4). From 10 months after the diagnosis of the disease, the probability of survival in people with primary metastasis decreased sharply until after 50 months. This probability reached zero in people without primary metastasis. The probability of survival declined, but after 50 months it remained at a constant rate of 50%. This relationship was significant with P=0.0116. Meanwhile, in individuals with primary metastasis, the probability of survival decreases until it reaches zero.
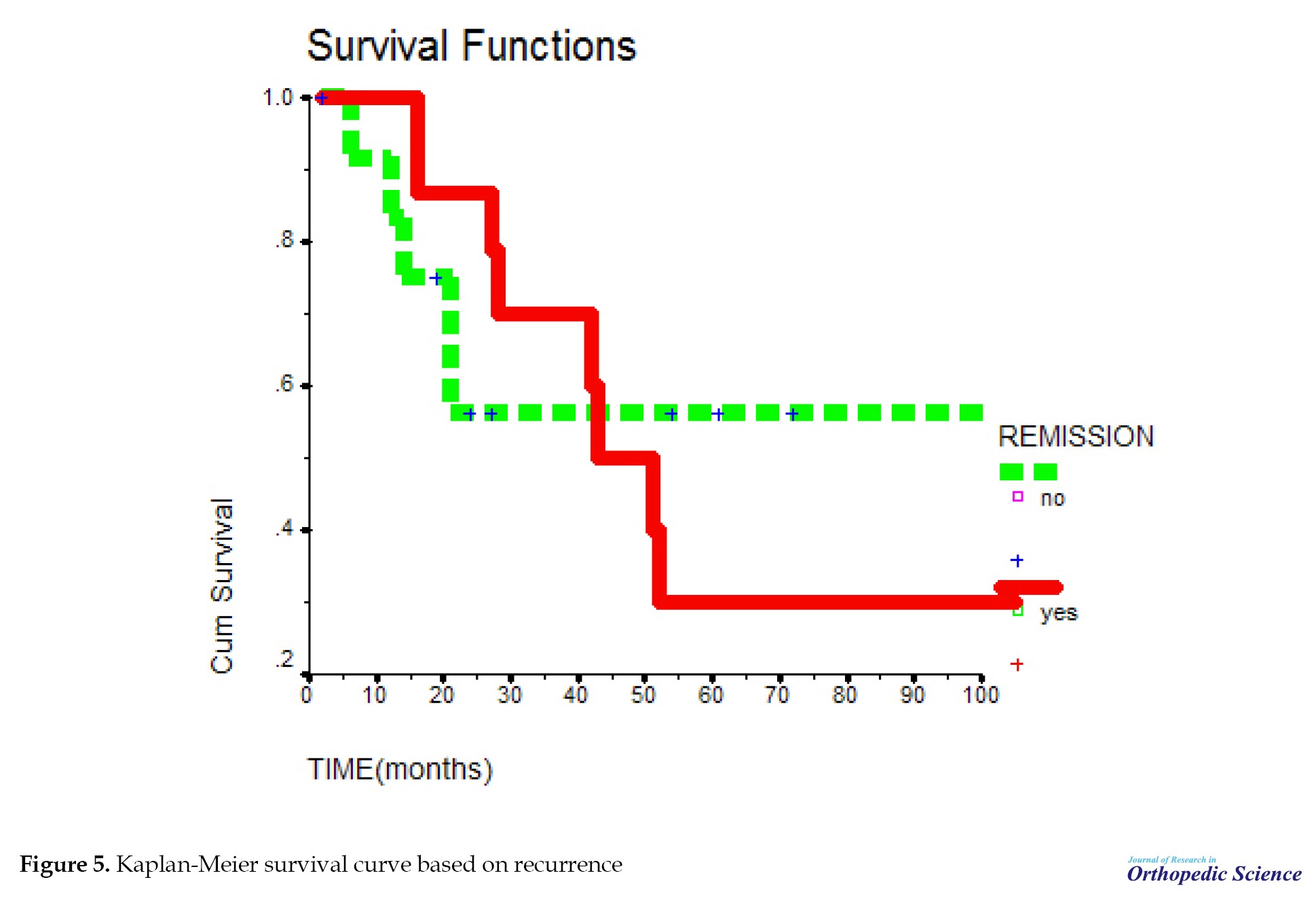
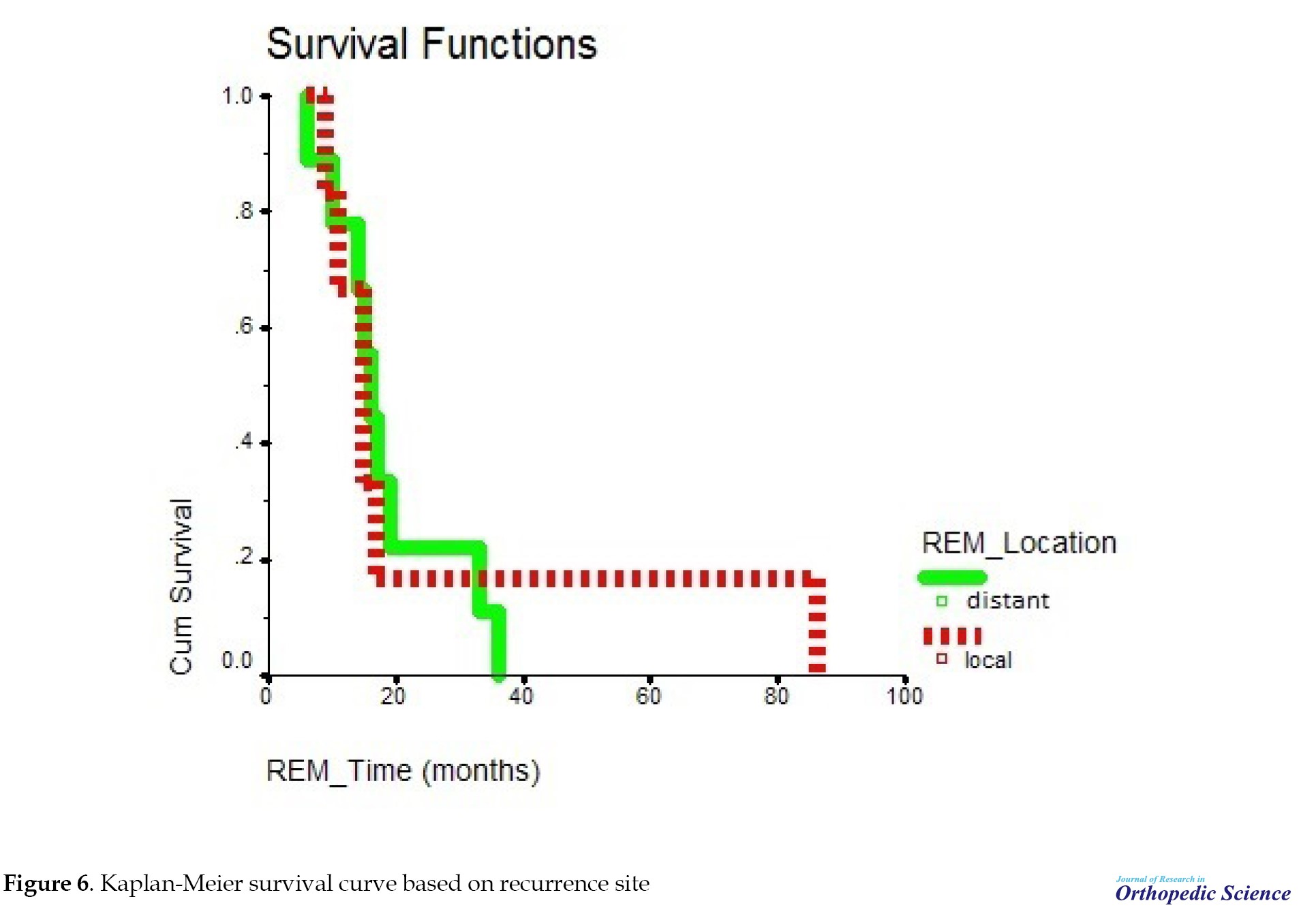
The mean survival time in people with recurrence was not significant compared to people without recurrence with P=0.9141 (Figure 5). After 20 months, the probability of survival of people without recurrence remained constant; however, in people with recurrence, the probability of survival declined and after 50 months, it stabilized (Figure 6). Cases in which osteosarcoma recurrence occurred in the primary bone were considered local recurrence. Meanwhile, cases in which the recurrence occurred elsewhere in the body other than the primary bone location were considered a distant recurrence. Among the people who had a recurrence, subjects who had a distant recurrence, the probability of survival decreased and after 38 months reached zero; however, in people with local recurrence, the probability of survival decreased and after 18 months it reached a constant rate of 18%. After 85 months, it reached zero. This relationship was not significant with P=0.8943.
4. Discussion
In this study, we performed a survival analysis of 29 patients with OS over 14 years. The mean survival period in the studied samples was 83.1±14.71. The mean survival period, according to sex, age, recurrence, and recurrence site, was not significant. However, the rates were significant according to metastasis status.
Local recurrence increases the risk of death in primary OS and indicates poor survival for patients [19]. However, this is not in line with our study. Ozger et al. conducted a study to analyze the survival time of OS patients and found that various factors, such as age, sex, and tumor location do not affect the survival time of patients, which is in line with our study [20].
Also, Aljubran et al. conducted a survival analysis of OS patients and stated that survival does not depend on age, and the 5-year survival rate was 66% without any difference between patients younger or older than 40 years old, which is in line with our findings [21]. Gelderblom et al. investigated survival after OS recurrence. They showed a relationship between early recurrence and poor survival, which is not in line with our results [22]. A study to determine prognostic factors for the survival of OS patients showed that survival depends on metastasis at the time of diagnosis, and 10-year survival is reduced in patients with primary metastasis, which is consistent with our study [23]. In the study of Dharanikota et al., similar to the present research, there were no significant differences in survival between age groups, sex of the patients, and status of local recurrence and absence of metastasis on follow-up independently predicted better overall survival. The 5-year overall survival rate of extremity osteosarcoma was 65.5% [24]. Yasin et al. showed that the 5-year overall survival rate was 56.3%, age, sex, and tumor location do not affect the survival time of patients. Two independent risk factors for survival were metastatic status and completion of treatment [25].
5. Conclusion
The mean survival time in OS patients does not depend on various factors, such as age, sex, tumor location, and recurrence site. However, primary metastasis reduces the mean survival time of patients.
Study limitations
This study was not without limitations. One of the main problems of our study was our small sample size, a larger sample size is suggested for future studies to better evaluate the effect of prognostic factors on the survival time of patients.
Ethical Considerations
Compliance with ethical guidelines
This study was reviewed and approved by the Ethics Committee of Islamic Azad University, Khorasgan branch (Code: IR.IAU.KHUISF.REC.1397.082) and patients provided written informed consent for participation.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Study design: Mohammad Reza Mortazavizadeh and Amir Ebrahimzadeh Babaki; Supervision: Mohammad Reza Mortazavizadeh; Data gathering and data analysis: Fahimeh Ebrahimzadeh Babaki; Drafting the manuscript: Amir Ebrahimzadeh Babak; Final approval: All authors.
Conflict of interest
All authors declared no conflict of interest.
References
Osteosarcoma (OS) is a malignant bone neoplasm originating from bone mesenchymal cells. It is the most common cause of limb loss due to cancers [1, 2]. It often affects children, teenagers, and young adults. OS has a bimodal distribution, most frequently affecting the youth and the elderly [2-4]. The incidence rate is estimated to be 3.4 per million people annually, and men are affected more than women [5]. This tumor originates in long bones, such as the distal femur, tibia, or humerus [3]. Approximately 15% to 20% of patients have metastases at the time of diagnosis. The lungs and bones are the most common sites of metastasis [2].
Before 1970, chemotherapy was not used for OS, and the survival rate was poor. When surgery was the only treatment method, most patients died one year after diagnosis, and the total 5-year survival rate was around 10% [6, 7]. The standard of care for OS is surgery with neoadjuvant and or adjuvant chemotherapy [6, 8, 9]. The four chemotherapy agents that are included in almost all treatment regimens include methotrexate, doxorubicin, cisplatin, and ifosfamide. Patients with metastasis may also be treated with etoposide [2, 10-13]. Tumor necrosis response to neoadjuvant chemotherapy is the overall response to treatment. Patients with tumor necrosis of more than 90% have a 5-year survival rate of 82%, while subjects with tumor necrosis of less than 90% have a 5-year survival rate of 68% [14]. The dose intensity is also important. In patients with localized OS, waiting more than 21 days after surgery to restart chemotherapy has a significantly high mortality rate. It is recommended to continue chemotherapy in the first 21 days after the operation to maintain the intensity of the dose [15].
Considering the lack of complete and definitive information regarding the choice of treatment based on the survival rate after treatment, this study examines the survival rate in patients with OS. Accordingly, we determine the mean survival time in patients with OS and associated factors.
2. Methods
This retrospective study was conducted on 29 patients with pathology-confirmed OS. Out of all the patients who were referred to the clinic, the number of patients to whom we had access and were able to check all their conditions was 29 patients. All patients who presented to the University Hospital (Shah Vali Hospital) in Yazd, Iran, from April 2004 to December 2018 were included in the study. The patients who could not be contacted were excluded from the study. Written informed consent was obtained from all patients before they participated in the study. Information on patient-related factors (age, sex), and tumor-related factors (recurrence, recurrence site, metastasis) were extracted from the hospital records, and information about living status was collected through phone calls and clinical visits.
We wanted to have an age division in this study to better evaluate adults and children. The age range of our patients was 8 to 42 years. In the fifth edition of the World Health Organization (WHO) classification, bone tumors are the most widely used pathologic classification system [16]. Bone cancers, such as osteosarcoma under the age of 20, are considered children’s cancers. Also, in some well-known articles and book chapters in this field [17, 18], the age group under 20 has been considered a separate group. Therefore, we divided our patients into two groups. Group 1 included the participants under the age of 20 years as children and the second group involved subjects over the age of 20 as adults.
The treatment process for most patients with osteosarcoma that has not metastasized was as follows: First, three courses of neoadjuvant chemotherapy were performed for the patient. Then, surgery was performed. After surgery, three courses of adjuvant chemotherapy were performed. For patients with metastasis, chemotherapy was performed, then surgery was performed if it could be done.
The chemotherapy regimen is cisplatin 70 mg + doxorubicin (adriamycin) 50 mg daily for three consecutive days every three weeks.
Surgery included resection with wide margins (removal of the tumor with a cuff of normal tissue covering it all around). This means the removal of 2 cm of normal tissue or a good anatomical barrier (e.g. fascial layer/articular cartilage) and osteotomy of bone 3-5 cm away from the level of involvement. In cases where large segmental defects were created following resection, reconstruction with a tumoral prosthesis was done. Meanwhile, in cases where the tumor could not be resected or the involvement on the surrounding tissues was aggressive or had caused neurovascular involvement, amputation was performed.
Data analysis
This study used the SPSS software, version 17 to analyze the data. The Kaplan-Meier test was used for survival analysis and the log-rank test was exploited to investigate the effect of risk factors, such as age, sex, local recurrence, and metastasis on patient survival.
3. Results
Overall, 29 patients were included in this study, of which 21(72.4%) were male (Table 1).

The mean age of patients was 21.3±8.4 (8-42) years (Table 2).

Meanwhile, 4 patients had tumors in the upper limb (all of them in the humerus), 23 patients in the lower limb (2 patients in the pelvis, 12 patients in the femur, and 9 patients in the tibia), and 2 patients had tumors in other areas (mandible and spine).
Thirteen (44.8%) patients died due to OS, and 1(6.9%) patient died due to other reasons (Table 3).

Regarding the type of treatment, 26(89.7%) patients underwent surgery, 26(89.7%) patients had adjuvant chemotherapy, and 27(93.1%) patients had neoadjuvant chemotherapy. The mean survival time in the subjects was 83.1±14.71 (95% CI, 54.27%, 111.92%) months. The median survival time was 51 months. The survival rate of patients for 1-year, 3-year, and 5-year periods was 90%, 64%, and 40%, respectively (Table 4).

The mean tumor necrosis rate was 79.53±28.84 (5%-100%). Meanwhile, 10 cases had a necrosis rate higher than 90%, and 5 patients had a tumor necrosis rate less than 90% (P=0.002). The necrosis rate was higher in the survivors.
The mean duration of disease-free survival was 38 months, and the patients’ mean survival time after relapse was 21.27±5.07 (95% CI, 11.33%, 31.21%) (Table 5).

The probability of survival decreased with a relatively sharp slope until 51 months, but from 51 months onward, the probability of survival remained almost constant and did not change. The mean survival time in the studied samples was 83.1. In addition, 1-year survival of patients was about 90%, 3-year survival was about 64%, and 5-year survival was 40% in the studied samples (Figure 1). The probability of survival was the same in both sexes. Survival time in this study was not related to gender (P=0.713) (Figure 2). The mean survival period at the ages of 8-19 years was not significant, compared to the ages of 20-42 years with P=0.4643 (Figure 3). The probability of survival up to 41 months was the same at different ages. After 41 months, the probability of survival remained constant at the ages of 20-42 years; however, at the ages of 8-19 years, the probability of survival decreased, but with the difference of P=0.46, which is not significant.
The mean survival time in people who had primary metastasis compared to people without primary metastasis was significant with P=0.0116; accordingly, primary metastasis causes a decrease in survival time (Figure 4). From 10 months after the diagnosis of the disease, the probability of survival in people with primary metastasis decreased sharply until after 50 months. This probability reached zero in people without primary metastasis. The probability of survival declined, but after 50 months it remained at a constant rate of 50%. This relationship was significant with P=0.0116. Meanwhile, in individuals with primary metastasis, the probability of survival decreases until it reaches zero.
The mean survival time in people with recurrence was not significant compared to people without recurrence with P=0.9141 (Figure 5). After 20 months, the probability of survival of people without recurrence remained constant; however, in people with recurrence, the probability of survival declined and after 50 months, it stabilized (Figure 6). Cases in which osteosarcoma recurrence occurred in the primary bone were considered local recurrence. Meanwhile, cases in which the recurrence occurred elsewhere in the body other than the primary bone location were considered a distant recurrence. Among the people who had a recurrence, subjects who had a distant recurrence, the probability of survival decreased and after 38 months reached zero; however, in people with local recurrence, the probability of survival decreased and after 18 months it reached a constant rate of 18%. After 85 months, it reached zero. This relationship was not significant with P=0.8943.
4. Discussion
In this study, we performed a survival analysis of 29 patients with OS over 14 years. The mean survival period in the studied samples was 83.1±14.71. The mean survival period, according to sex, age, recurrence, and recurrence site, was not significant. However, the rates were significant according to metastasis status.
Local recurrence increases the risk of death in primary OS and indicates poor survival for patients [19]. However, this is not in line with our study. Ozger et al. conducted a study to analyze the survival time of OS patients and found that various factors, such as age, sex, and tumor location do not affect the survival time of patients, which is in line with our study [20].
Also, Aljubran et al. conducted a survival analysis of OS patients and stated that survival does not depend on age, and the 5-year survival rate was 66% without any difference between patients younger or older than 40 years old, which is in line with our findings [21]. Gelderblom et al. investigated survival after OS recurrence. They showed a relationship between early recurrence and poor survival, which is not in line with our results [22]. A study to determine prognostic factors for the survival of OS patients showed that survival depends on metastasis at the time of diagnosis, and 10-year survival is reduced in patients with primary metastasis, which is consistent with our study [23]. In the study of Dharanikota et al., similar to the present research, there were no significant differences in survival between age groups, sex of the patients, and status of local recurrence and absence of metastasis on follow-up independently predicted better overall survival. The 5-year overall survival rate of extremity osteosarcoma was 65.5% [24]. Yasin et al. showed that the 5-year overall survival rate was 56.3%, age, sex, and tumor location do not affect the survival time of patients. Two independent risk factors for survival were metastatic status and completion of treatment [25].
5. Conclusion
The mean survival time in OS patients does not depend on various factors, such as age, sex, tumor location, and recurrence site. However, primary metastasis reduces the mean survival time of patients.
Study limitations
This study was not without limitations. One of the main problems of our study was our small sample size, a larger sample size is suggested for future studies to better evaluate the effect of prognostic factors on the survival time of patients.
Ethical Considerations
Compliance with ethical guidelines
This study was reviewed and approved by the Ethics Committee of Islamic Azad University, Khorasgan branch (Code: IR.IAU.KHUISF.REC.1397.082) and patients provided written informed consent for participation.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Study design: Mohammad Reza Mortazavizadeh and Amir Ebrahimzadeh Babaki; Supervision: Mohammad Reza Mortazavizadeh; Data gathering and data analysis: Fahimeh Ebrahimzadeh Babaki; Drafting the manuscript: Amir Ebrahimzadeh Babak; Final approval: All authors.
Conflict of interest
All authors declared no conflict of interest.
References
- Settakorn J, Rangdaeng S, Arpornchayanon O, Lekawanvijit S, Bhoopat L, Attia J. Why were limbs amputated? An evaluation of 216 surgical specimens from Chiang Mai University Hospital, Thailand. Arch Orthop Trauma Surg. 2005; 125(10):701-5 [DOI:10.1007/s00402-005-0060-y] [PMID]
- Pruksakorn D, Phanphaisarn A, Arpornchayanon O, Uttamo N, Leerapun T, Settakorn J. Survival rate and prognostic factors of conventional osteosarcoma in Northern Thailand: A series from Chiang Mai University Hospital. Cancer Epidemiol. 2015; 39(6):956-63. [DOI:10.1016/j.canep.2015.10.016] [PMID]
- Mirabello L, Troisi RJ, Savage SA. International osteosarcoma incidence patterns in children and adolescents, middle ages and elderly persons.Int J Cancer. 2009; 125(1):229-34. [DOI:10.1002/ijc.24320] [PMID]
- Damron TA, Ward WG, Stewart A. Osteosarcoma, chondrosarcoma, and Ewing’s sarcoma: National Cancer Data Base Report. Clin Orthop Relat Res. 2007; 459:40-7. [DOI:10.1097/BLO.0b013e318059b8c9] [PMID]
- Brawley OW. The American Cancer Society and the American health care system. Oncologist. 2011; 16(7):920-5. [DOI:10.1634/theoncologist.2011-0074] [PMID]
- Imura Y, Takenaka S, Kakunaga S, Nakai T, Wakamatsu T, Outani H, et al. Survival analysis of elderly patients with osteosarcoma. Int Orthop. 2019; 43(7):1741-7. [DOI:10.1007/s00264-019-04332-y] [PMID]
- Bacci G, Picci P, Ferrari S, Ruggieri P, Casadei R, Tienghi A, et al. Primary chemotherapy and delayed surgery for nonmetastatic osteosarcoma of the extremities. Results in 164 patients preoperatively treated with high doses of methotrexate followed by cisplatin and doxorubicin. Cancer. 1993; 72(11):3227-38. [DOI:10.1002/1097-0142(19931201)72:113.0.CO;2-C] [PMID]
- Bielack SS, Kempf-Bielack B, Delling G, Exner GU, Flege S, Helmke K, et al. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol. 2002; 20(3):776-90. [DOI:10.1200/JCO.2002.20.3.776] [PMID]
- Meyers PA, Schwartz CL, Krailo M, Kleinerman ES, Betcher D, Bernstein ML, et al. Osteosarcoma: A randomized, prospective trial of the addition of ifosfamide and/or muramyl tripeptide to cisplatin, doxorubicin, and high-dose methotrexate. J Clin Oncol. 2005; 23(9):2004-11. [DOI:10.1200/JCO.2005.06.031] [PMID]
- Jaffe N. Osteosarcoma: Review of the past, impact on the future. The American experience. Cancer Treat Res. 2009; 152:239-62. [DOI:10.1007/978-1-4419-0284-9_12] [PMID]
- Whelan JS, Bielack SS, Marina N, Smeland S, Jovic G, Hook JM, et al. EURAMOS-1, an international randomised study for osteosarcoma: Results from pre-randomisation treatment. Ann Oncol. 2015; 26(2):407-14. [DOI:10.1093/annonc/mdu526] [PMID]
- Anninga JK, Gelderblom H, Fiocco M, Kroep JR, Taminiau AH, Hogendoorn PC, et al. Chemotherapeutic adjuvant treatment for osteosarcoma: Where do we stand? Eur J Cancer. 2011; 47(16):2431-45. [DOI:10.1016/j.ejca.2011.05.030] [PMID]
- Eselgrim M, Grunert H, Kühne T, Zoubek A, Kevric M, Bürger H, et al. Dose intensity of chemotherapy for osteosarcoma and outcome in the Cooperative Osteosarcoma Study Group (COSS) trials. Pediatr Blood Cancer. 2006; 47(1):42-50. [DOI:10.1002/pbc.20608] [PMID]
- Lewis VO, Gebhardt MC, Springfield DS. Parosteal osteosarcoma of the posterior aspect of the distal part of the femur.Oncological and functional results following a new resection technique. J Bone Joint Surg Am. 2000; 82(8):1083-8. [DOI:10.2106/00004623-200008000-00003] [PMID]
- Kapoor S, Tiwari A, Kapoor S. Primary tumours and tumorous lesions of clavicle. Int Orthop. 2008; 32(6):829-34.[DOI:10.1007/s00264-007-0397-7] [PMID]
- WHO Classification of Tumours Editorial Board. Soft Tissue and Bone Tumours, WHO Classification of Tumours, 5th Edition, Volume 3. Lyon: IARC; 2020. [Link]
- Ottaviani G, Jaffe N. The epidemiology of osteosarcoma. Cancer Treat Res. 2009; 152:3-13. [DOI:10.1007/978-1-4419-0284-9_1] [PMID]
- Janeway KA, Gorlick R, Bernstein ML. 22 - Osteosarcoma. Oncology of Infancy and Childhood. 2009; 871-910. [DOI:10.1016/B978-1-4160-3431-5.00022-4]
- Weeden S, Grimer RJ, Cannon SR, Taminiau AH, Uscinska BM; European Osteosarcoma Intergroup. The effect of local recurrence on survival in resected osteosarcoma. Eur J Cancer. 2001; 37(1):39-46. [DOI:10.1016/S0959-8049(00)00362-2] [PMID]
- Ozger H, Eralp L, Atalar AC, Toker B, Ayan I, Kebudi R, et al. [Survival analysis and the effects of prognostic factors in patients treated for osteosarcoma (Turkish)]. Acta Orthop Traumatol Turc. 2007; 41(3):211-9. [PMID]
- Aljubran AH, Griffin A, Pintilie M, Blackstein M. Osteosarcoma in adolescents and adults: Survival analysis with and without lung metastases. Ann Oncol. 2009; 20(6):1136-41. [DOI:10.1093/annonc/mdn731] [PMID]
- Gelderblom H, Jinks RC, Sydes M, Bramwell VH, van Glabbeke M, Grimer RJ, et al. Survival after recurrent osteosarcoma: Data from 3 European Osteosarcoma Intergroup (EOI) randomized controlled trials. Eur J Cancer. 2011; 47(6):895-902. [DOI:10.1016/j.ejca.2010.11.036] [PMID]
- Duchman KR, Gao Y, Miller BJ. Prognostic factors for survival in patients with high-grade osteosarcoma using the Surveillance, Epidemiology, and End Results (SEER) Program database. Cancer Epidemiol. 2015; 39(4):593-9.[DOI:10.1016/j.canep.2015.05.001] [PMID]
- Dharanikota A, Arjunan R, Dasappa A. Factors affecting prognosis and survival in extremity osteosarcoma. Indian J Surg Oncol. 2021; 12(1):199-206. [DOI:10.1007/s13193-020-01277-2] [PMID]
- Yasin NF, Abdul Rashid ML, Ajit Singh V. Survival analysis of osteosarcoma patients: A 15-year experience. J Orthop Surg (Hong Kong). 2020; 28(1):2309499019896662.[DOI:10.1177/2309499019896662] [PMID]
Type of Study: Research Article |
Subject:
Tumor surgery
Received: 2022/03/25 | Accepted: 2022/04/29 | Published: 2022/11/1
Received: 2022/03/25 | Accepted: 2022/04/29 | Published: 2022/11/1
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |