Volume 10, Issue 1 (2-2023)
JROS 2023, 10(1): 27-34 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Moghtadaei M, Yeganeh A, Amin Pirjeli S, Farahini H. Effect of Systemic Corticosteroids on Pain Control After Total Knee Arthroplasty. JROS 2023; 10 (1) :27-34
URL: http://jros.iums.ac.ir/article-1-2246-en.html
URL: http://jros.iums.ac.ir/article-1-2246-en.html
1- Department of Orthopedics, School of Medicine, Bone and Joint Reconstruction Research Center, Iran University of Medical Sciences, Tehran, Iran.
Full-Text [PDF 912 kb]
(256 Downloads)
| Abstract (HTML) (741 Views)
Full-Text: (304 Views)
Introduction
Total knee arthroplasty (TKA) improves the function and pain of people suffering from knee osteoarthritis. Patient satisfaction one year after TKA is between 75% and 89.8% [1]. As a result, TKA is one of the most common elective orthopedic procedures. Despite the long-term success of arthroplasty, the postoperative experience of patients is unpleasant due to nausea, vomiting, and pain [2]. One of the prominent characteristics of patients after TKA is severe pain in the first postoperative stage. About 60% of the patients have severe pain, and 30% have moderate pain after the operation [3, 4]. This unpleasant experience not only reduces patient satisfaction but can also reduce patient participation in physical therapy. Therefore, it increases the length of hospital stay and the use of painkillers and anti-nausea drugs [5]. Pain reduction is necessary for faster recovery, reaching patients’ optimal range of motion, and obtaining better results [6].
Studies have shown that preoperative systemic glucocorticoid injection reduces postoperative nausea and vomiting (PONV) and pain in patients. The addition of glucocorticoids to the patient’s pain medication can reduce the amount of medication used for nausea and pain in the postoperative period [7]. Theoretically, in patients undergoing TKA, the reduction of PONV and pain should lead to mobilization and early discharge from the hospital. The benefits of using glucocorticoids, including reducing pain, improving patient satisfaction, and reducing resource use, should be weighed against the potential risks of their use [8].
Unpleasant complications that occur after TKA include infection and wound complications. Long-term use of glucocorticoids increases the risk of these complications. The risk of complications from the limited use of glucocorticoids in knee and hip arthroplasty surgery is not clearly defined [8]. Although multiple pain control methods and various surgical and anesthetic methods are used to manage these patients, pain is still one of the major problems of these patients after surgery [8, 9].
Objectives
Considering the importance of this issue, the present study was designed to evaluate the effect of systemic corticosteroids in controlling pain after knee joint replacement. The findings of this study can help choose the best treatment method to control pain in patients undergoing knee joint replacement surgery.
Methods
The present study was designed as a randomized parallel controlled clinical trial. The study population included all patients who were candidates for joint replacement surgery and underwent surgery in three medical centers of Hazrat-e-Rasool Akram (PBUH), Moheb, and Chamran between September 2022 and October 2023. The study was performed after the Ethics Committee of the Iran University of Medical Sciences approved it. All patients read and signed the informed consent form before entering this study. The inclusion criteria include age range from 40 to 80 years, physical status classification of ASA 1 to ASA 3, based on the American Society of Anesthesiologists (ASA), no fracture in other knee bones, no history of knee surgery, cooperation to follow up patients, no drug addiction, and informed consent to participate in the study. The exclusion criteria included contraindications for local anesthesia, coagulation disorder, platelet level of less than 100000, history of chronic kidney failure with glomerular filtration rate (GFR) <60 mL/min, non-cooperation or unwillingness to participate in the study.
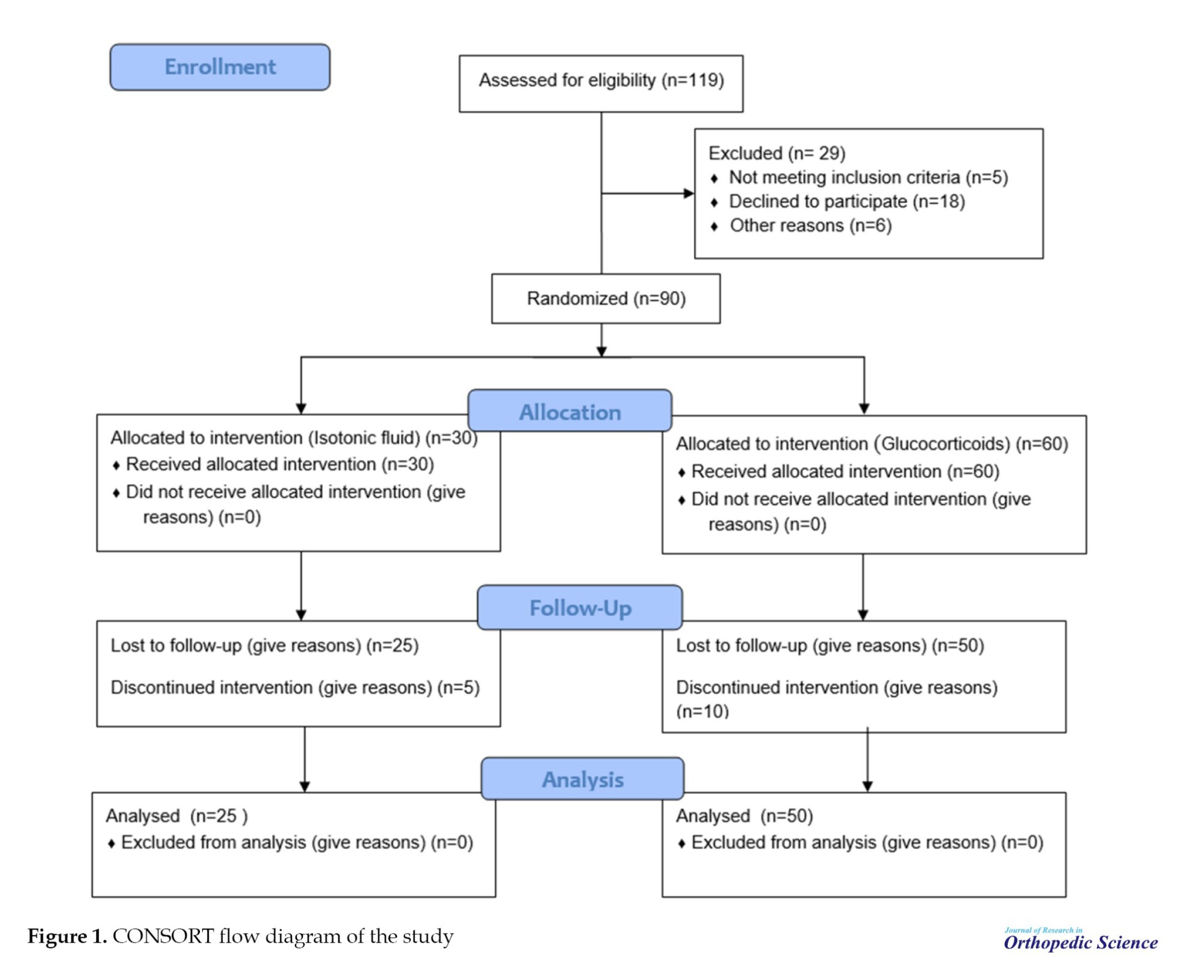
Based on the Cochran formula and similar studies, a sample size of 42 people was obtained, and to increase the power of the study, 90 patients were included in the study. Figure 1 shows the consolidated standards of reporting trials (CONSORT) study flow diagram. The patients were diagnosed randomly and put in equal proportions to three groups. The first group (dexamethasone [Dex]) consisted of 25 patients who received an 8 mg dose of Dex intravenously in three doses of 24 mg/IV. The second group (methylprednisolone [Met]) included 25 patients who received Met (Depo-Medrol) intravenously at a dose of 125 mg, and the control group (Placebo) included 25 patients who received 2 mL isotonic fluid intravenously. The study participants and the person injecting the drug and placebo were blind to what drug they received or injected.
All patients underwent the usual surgical procedures, anesthesia, and analgesia. Approximately 1-2 hours before surgery, patients received oral gabapentin 600 mg, paracetamol in slow-release form 2 g, and celecoxib 400 mg. All patients undertook lumbar spinal anesthesia with 10 mg hyperbaric bupivacaine. Propofol (1-5 mg/kg/h) was ordered if necessary.
Primary outcomes included knee pain 4, 12, and 24 hours after surgery as assessed by a walker using a visual analog scale (VAS, 0 no pain and 100, worst imaginable pain). Similarly, pain at rest was assessed as a secondary outcome. Pain was evaluated 4, 12, and 24 hours after surgery [10]. Nausea was evaluated using a 4-point numerical scale (0: None, 1: Mild, 2: Moderate, 3: Severe) and the number of vomiting episodes [11]. In addition, patients’ performance indices after surgery were evaluated by the Western Ontario and McMaster universities osteoarthritis (WOMAC) index. The test questions are scored from 0 to 4 (0: None, 1: Mild, 2: Moderate, 3: Severe, and 4: Extreme). The scores for each subscale are summed up, with a possible score range of 0-68 for physical functions, 0-20 for pain, and 0-8 for stiffness [12].
To assess the knee’s range of motion, the subject lay comfortably on the examination bed, and the leg was placed flat. The axis of the goniometer was placed in the outer part of the knee on the tibial condyle, and the fixed arm was placed in the outer part of the thigh parallel to the longitudinal axis of the thigh. The movable arm was placed in the outer part of the leg parallel to the longitudinal axis of the tibia. The subject then flexed the knee, and the amount of flexion was measured. At the follow-up examination 6 weeks after the operation, the average pain during the day, in walking and at rest, was recorded [13]. In addition, the average degree of vomiting and nausea during the day, the quality of sleep at night, fatigue during the day, the daily intake of pain medication (type and dose), sleep medication (yes or no), anti-nausea medication (yes or no), side effects and infection were recorded. In addition, side effects, infections, and complications during hospitalization were recorded at the follow-up visit 6 weeks after the surgery. After collecting the information, all data were analyzed using SPSS software, version 16. The descriptive information was presented as Mean±SD or percentage. The Shapiro-Wilk test was used to assess the normality of the data. The t-test was used to compare quantitative variables in two groups with the assumption of normal distribution. A one-way test was used to compare quantitative variables in more than two groups. If the distribution of the variables was not normal, the non-parametric Mann-Whitney or Kruskal-Wallis tests were used. The paired t-test or its non-parametric equivalent (Wilcoxon signed-rank test) was used to compare the variables before and after the intervention. The chi-square test was used to compare qualitative variables. The repeated measures analysis of variance (ANOVA) was used to compare slightly repeated variables. P<0.05 were considered significant.
Results
In general, 75 patients (25 in each group) completed the study. Demographic characteristics in the control, Met, and Dex are summarized in Table 1. No significant difference was reported for any demographic variables in the three groups at the beginning of the study (P>0.05).
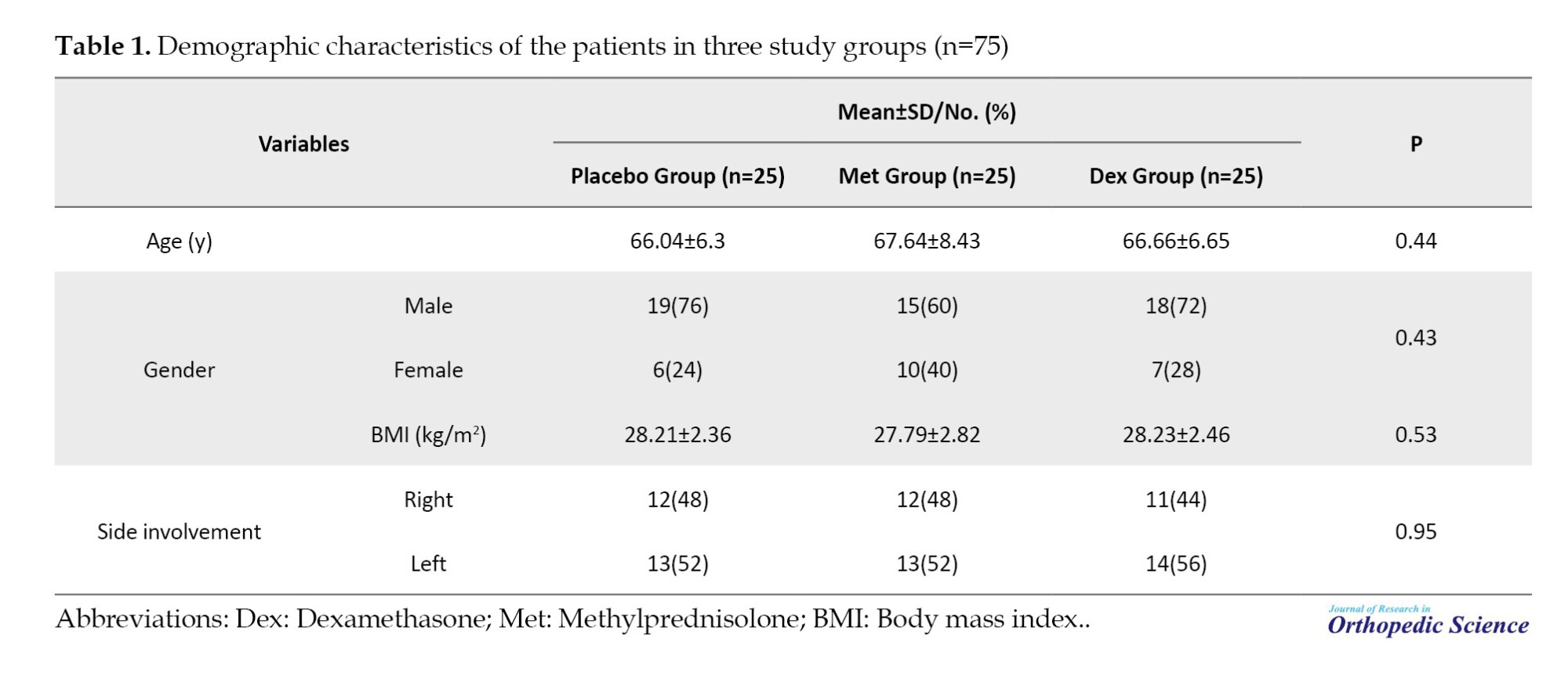
Table 2 compares the VAS scores in the three groups, showing significant differences at 4, 12, and 24 hours after the operation (P<0.001).
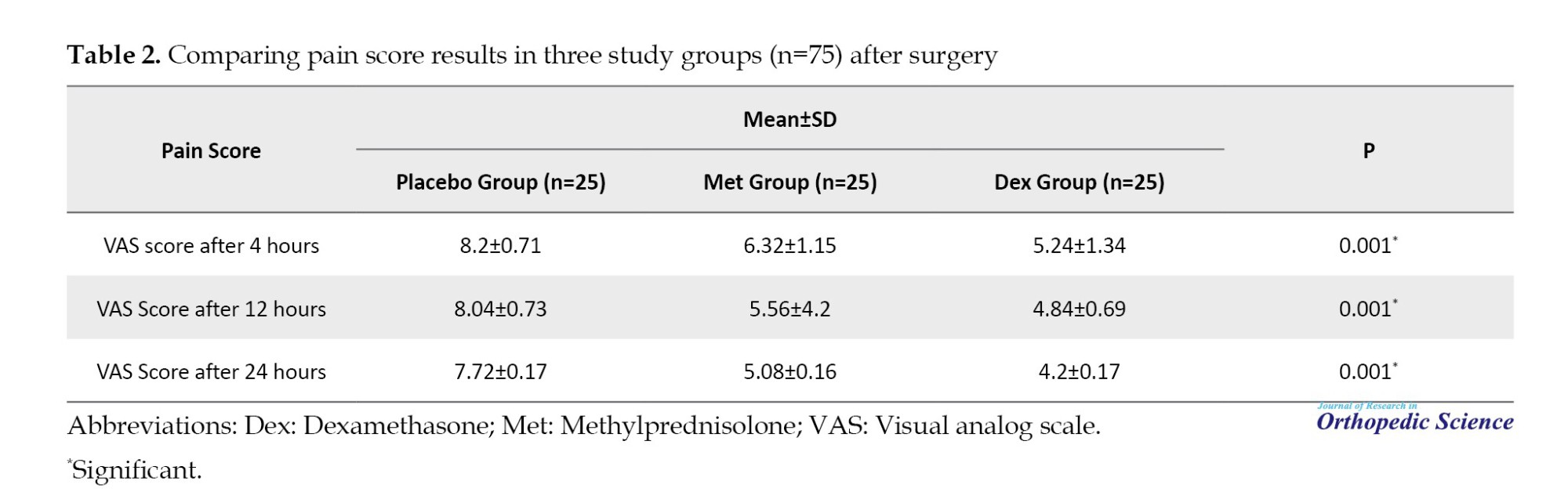
Figure 2 compares the mean score of the VAS in the three groups of study 4, 12, and 24 h after the operation.
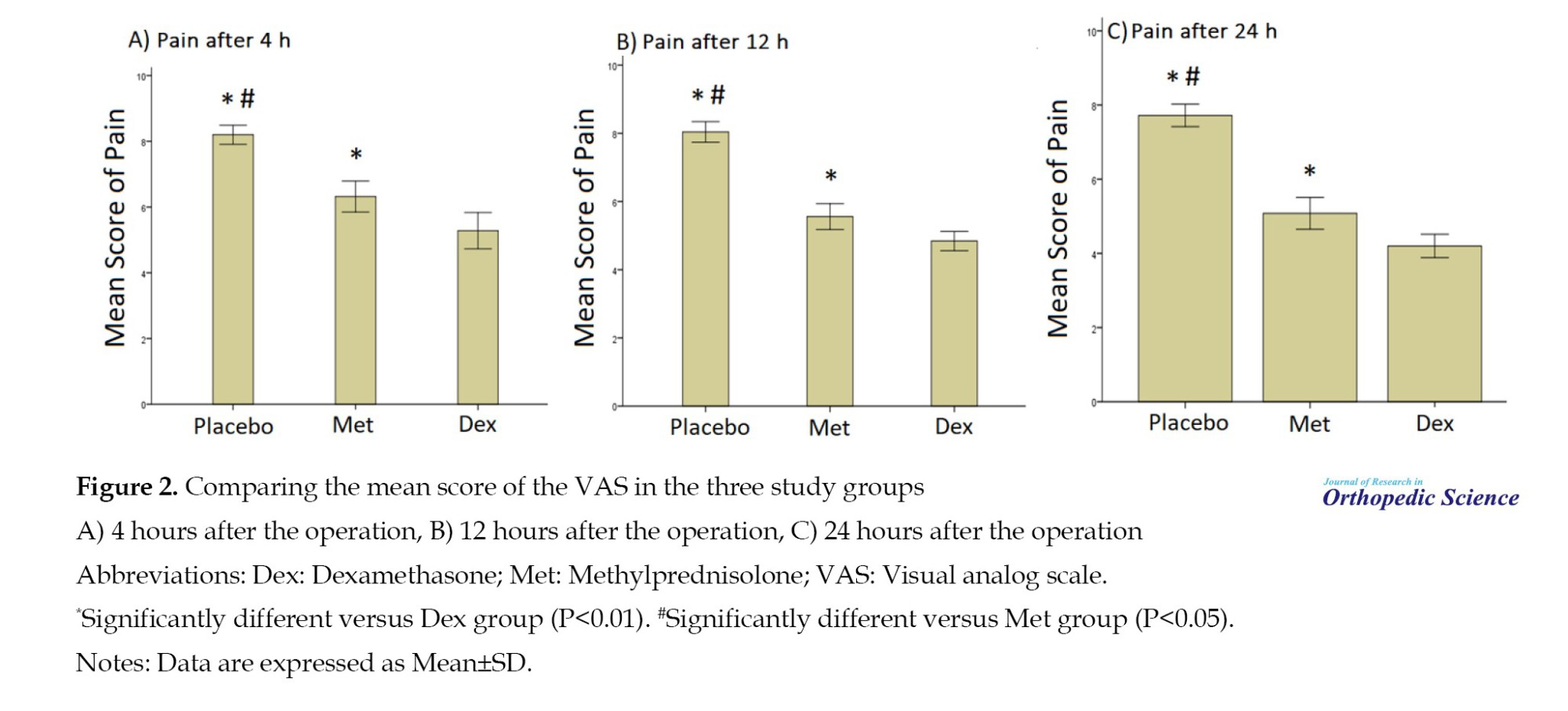
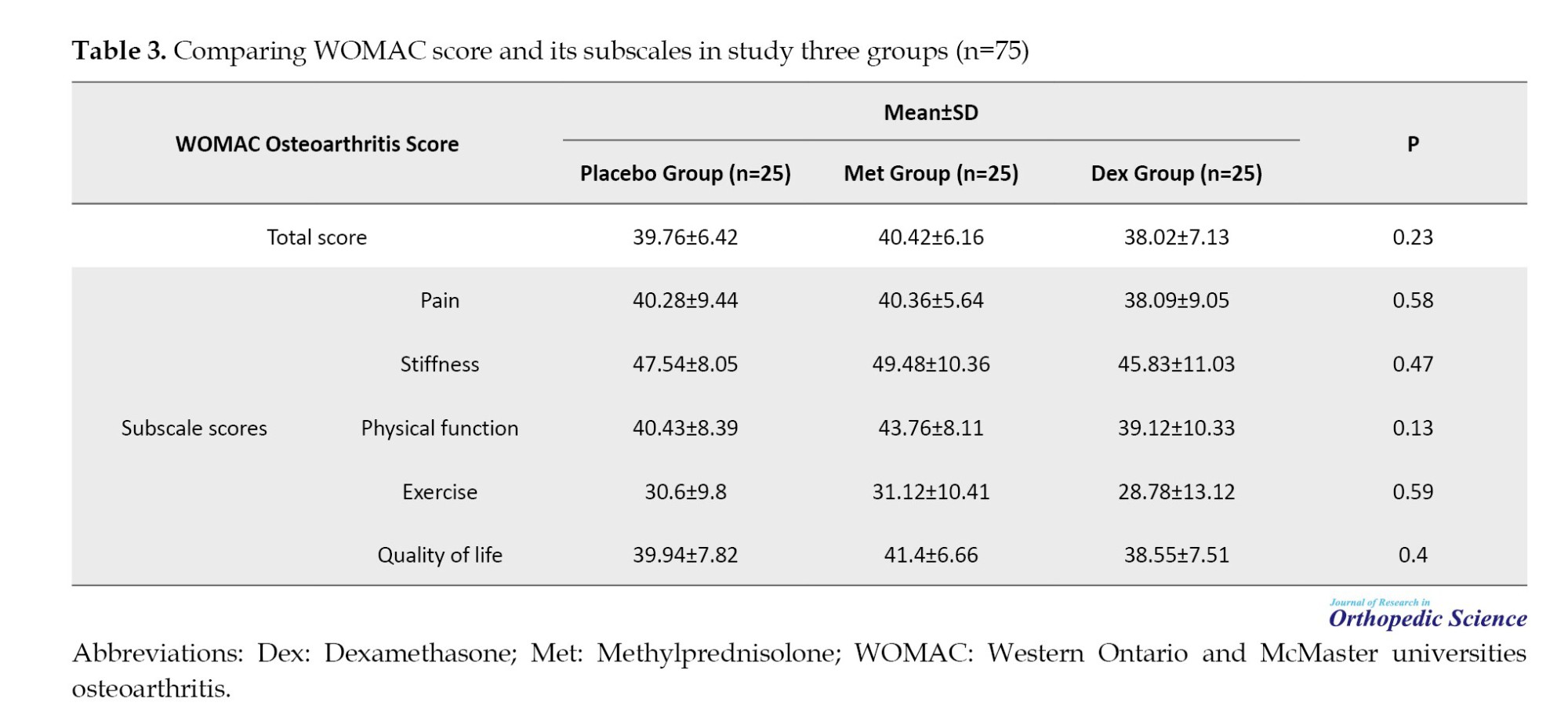
Table 3 compares the WOMAC score between the Dex, Met, and placebo groups, which was not significantly different. Also, no significant difference was observed for WOMAC subscales in the three groups after treatment (P>0.05).
In terms of postoperative complications, the finding showed that postoperative nausea was reported in 16 patients (21.3%). The frequency of nausea for the placebo, Met, and Dex groups was 9(36%), 6(24%), and 1(4%), respectively, and the difference was statistically significant (P=0.002). No other complications were reported after the operation in the three groups. The result showed the range of motion was normal in all patients after surgery.
Discussion
In this study, we evaluated the effect of systemic corticosteroids on pain control after TKA. Reducing pain is the most important goal of knee joint replacement and is the demand of patients. Corticosteroids are applied to relieve inflammation of the joints and different body parts or as part of the treatment process for various diseases (such as severe allergies, skin problems, asthma, etc.) with two anti-inflammatory and immunosuppressive mechanisms in treating the disease. The risk of complications from the limited use of glucocorticoids in knee and hip arthroplasty surgery is not clearly defined [9]. Although multiple pain control methods and various surgical and anesthetic methods are used to manage these patients, pain is still one of the major problems of these patients after surgery [14, 15].
According to the results of the present study, most patients were female candidates for joint replacement surgery. The majority of patients underwent surgery in their sixth and seventh decades of life, so the mean age of these patients was 66. Most patients were overweight and obese, and their mean body mass index (BMI) was 28.07 kg/m2; obesity is associated with an increase in arthritis in the joints. Three treatment groups were completely homogeneous regarding demographic characteristics at the beginning of the study, and no significant difference was reported for any demographic variables in the three groups at the start of the study. The mean score of VAS in the three groups, 4, 12, and 24 hours after the operation, was significantly different in the treatment groups. In all time intervals evaluated after the operation, the pain intensity in the Dex user group was significantly lower than in the Met and placebo groups. Twenty-four hours after the operation, the mean pain score was significantly lower in the Dex and Met groups than in the placebo group. Also, the mean pain score 24 hours after the operation in the Dex group was significantly lower than in the Met group. No significant differences were observed for the mean WOMAC score and its subscales after surgery in the three groups. In other words, the use of corticosteroids after surgery was only related to pain control 24 hours after surgery.
Nausea was the most common complication after the operation and had the highest frequency in the placebo group and the lowest frequency in the Dex group, and this difference was statistically significant. In other words, Dex improved postoperative pain control and nausea. The results of our study are consistent with the other studies conducted in this field [16-21]. In line with the results of our research, in a clinical trial study, Kim et al. evaluated the effectiveness of systemic steroid use in reducing pain and nausea one day after TKA. They showed that the pain intensity in patients who received 0.1 mL Dex gram was significantly less than in the control group. Also, the severity of pain and nausea in another group of patients who received 0.2 mg/kg Dex 24 hours after surgery was lower than the control group and the group of patients who received 0.1 mg/kg Dex, which confirmed these results. In the study, similar to our study, no complications were reported in the groups. They showed that administering Dex before and after the operation was effective for controlling pain and nausea 24 hours after the operation [16], which is in line with the results of our study. In our study, the functional indicators, as well as the range of motion of the patients, were evaluated, and no significant difference was observed for these indicators in the groups, which shows that the administration of corticosteroids is only effective for reducing acute outcomes after surgery, such as pain control and reducing nausea.
In a meta-analysis, Liang et al. investigated the effect of intravenous Dex on postoperative pain control in patients undergoing TKA and showed that the addition of postoperative intravenous Dex for postoperative pain control in TKA significantly reduces analgesic use, the length of stay in the hospital and pain [17] and the systematic review confirmed our results. In the present research, the effect of Dex on reducing the length of hospital stay was not evaluated. Still, it is expected that with the decrease in pain intensity in patients, the length of hospital stay will also decrease in this group of patients. In another review paper, Liu et al. evaluated the effect of intravenous glucocorticoids before surgery in reducing acute pain after TKA, which examined 11 RCT studies, including 1000 patients (glucocorticoids=501, control=499). The results showed that preoperative intravenous glucocorticoids significantly reduced the VAS score for pain 6, 12, 24, and 48 hours after surgery. In addition, the use of these drugs was associated with a significant reduction of 19.4% and 16.8% in the incidence of nausea and vomiting, which was consistent with the results of our study. Our study shows intravenous glucocorticoids reduce postoperative pain and PONV in patients after TKA. However, more studies are needed to determine the maximum dose and type of glucocorticoids for better pain control in these patients [18]. Our study shows that administering Dex in lower doses, compared to other studies, effectively reduces pain in these patients without causing complications. In this regard, a study by Kardash et al. examined whether administering a single dose of Dex lowers dynamic pain after total hip arthroplasty in 50 patients. They reported that postoperative pain in the group Dex, as well as the need for painkillers and sedatives, decreased significantly [19]. In another study by Lunn et al., the effectiveness of Met 125 mg before surgery on pain and recovery after TKA was reported. It has a positive effect on pain control and recovery after TKA. Also, no side effects were observed in the study [20], which was in line with the results of our study. In our study, the use of Met was significantly associated with a reduction in postoperative pain and nausea compared to the control group. While the amount of pain reduction and nausea after surgery was lower for Met than for Dex. In another study, Backes et al. examined 120 patients who underwent knee and hip arthroplasty and reported that intravenous injection of 10 mg of Dex significantly reduced the prescription of anti-nausea and analgesic drugs and the duration of hospitalization compared to the control group [21]. In another study, Lex et al. concluded that intraoperative corticosteroids are safe, reduce postoperative pain, and accelerate patient recovery after unilateral TKA and total hip arthroplasty [22] in accordance with our study’s results.
Conclusion
This study shows that administering 24 mg of Dex and 125 mg of Met significantly reduces pain intensity and nausea 6, 12, and 24 hours after TKA. Met is significantly less effective than Dex in controlling pain and nausea. The administration of Dex and Met did not affect improvement based on the WOMAC index in patients after surgery. Given that nausea and pain persist for more than 24 hours after TKA, additional administration of Dex for a longer postoperative period should be considered. Considering the harmful effects of long-term use of these drugs with high doses, these results can be justified, and there is still a need to design and conduct clinical trial studies to determine the safe and effective dose of these drugs; it is felt that the present study is also the same.
Our study had some limitations that should be noted. The most important weakness of our study was the lack of estimation of some outcomes (such as duration of stay in hospital) due to the prevalence of the COVID-19 pandemic during the study. Moreover, with the spread of the COVID-19 pandemic, elective surgeries in medical centers decreased. The most important strength of the present study was the design of the study as a randomized controlled clinical trial with an appropriate sample size and the examination of the results in three intervention groups in parallel after the operation.
Ethical Considerations
Compliance with ethical guidelines
The present study followed the Declaration of Helsinki and the Ethical Guidelines for Medical and Health Research established by the Ministry of Health and Medical Education and the Ministry of Science, Research and Technology, Iran. This study obtained the approval by the Ethics Review Committee of Iran University of Medical Sciences, Tehran, Iran (Code: IR.IUMS.FMD.REC.1399.014). Informed consent was obtained from each patient included in the study.
Funding
The current research was funded by a specific project grant from the Iran University of Medical Sciences.
Authors' contributions
Conceptualization and supervision: Mehdi Moghtadaei, and Hossein Farahini; Methodology: Ali Yeganeh; Data collection and analysis: Sajad Amin Pirjeli; Investigation and writing: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to thank Iran University of Medical Sciences.
References
Total knee arthroplasty (TKA) improves the function and pain of people suffering from knee osteoarthritis. Patient satisfaction one year after TKA is between 75% and 89.8% [1]. As a result, TKA is one of the most common elective orthopedic procedures. Despite the long-term success of arthroplasty, the postoperative experience of patients is unpleasant due to nausea, vomiting, and pain [2]. One of the prominent characteristics of patients after TKA is severe pain in the first postoperative stage. About 60% of the patients have severe pain, and 30% have moderate pain after the operation [3, 4]. This unpleasant experience not only reduces patient satisfaction but can also reduce patient participation in physical therapy. Therefore, it increases the length of hospital stay and the use of painkillers and anti-nausea drugs [5]. Pain reduction is necessary for faster recovery, reaching patients’ optimal range of motion, and obtaining better results [6].
Studies have shown that preoperative systemic glucocorticoid injection reduces postoperative nausea and vomiting (PONV) and pain in patients. The addition of glucocorticoids to the patient’s pain medication can reduce the amount of medication used for nausea and pain in the postoperative period [7]. Theoretically, in patients undergoing TKA, the reduction of PONV and pain should lead to mobilization and early discharge from the hospital. The benefits of using glucocorticoids, including reducing pain, improving patient satisfaction, and reducing resource use, should be weighed against the potential risks of their use [8].
Unpleasant complications that occur after TKA include infection and wound complications. Long-term use of glucocorticoids increases the risk of these complications. The risk of complications from the limited use of glucocorticoids in knee and hip arthroplasty surgery is not clearly defined [8]. Although multiple pain control methods and various surgical and anesthetic methods are used to manage these patients, pain is still one of the major problems of these patients after surgery [8, 9].
Objectives
Considering the importance of this issue, the present study was designed to evaluate the effect of systemic corticosteroids in controlling pain after knee joint replacement. The findings of this study can help choose the best treatment method to control pain in patients undergoing knee joint replacement surgery.
Methods
The present study was designed as a randomized parallel controlled clinical trial. The study population included all patients who were candidates for joint replacement surgery and underwent surgery in three medical centers of Hazrat-e-Rasool Akram (PBUH), Moheb, and Chamran between September 2022 and October 2023. The study was performed after the Ethics Committee of the Iran University of Medical Sciences approved it. All patients read and signed the informed consent form before entering this study. The inclusion criteria include age range from 40 to 80 years, physical status classification of ASA 1 to ASA 3, based on the American Society of Anesthesiologists (ASA), no fracture in other knee bones, no history of knee surgery, cooperation to follow up patients, no drug addiction, and informed consent to participate in the study. The exclusion criteria included contraindications for local anesthesia, coagulation disorder, platelet level of less than 100000, history of chronic kidney failure with glomerular filtration rate (GFR) <60 mL/min, non-cooperation or unwillingness to participate in the study.
Based on the Cochran formula and similar studies, a sample size of 42 people was obtained, and to increase the power of the study, 90 patients were included in the study. Figure 1 shows the consolidated standards of reporting trials (CONSORT) study flow diagram. The patients were diagnosed randomly and put in equal proportions to three groups. The first group (dexamethasone [Dex]) consisted of 25 patients who received an 8 mg dose of Dex intravenously in three doses of 24 mg/IV. The second group (methylprednisolone [Met]) included 25 patients who received Met (Depo-Medrol) intravenously at a dose of 125 mg, and the control group (Placebo) included 25 patients who received 2 mL isotonic fluid intravenously. The study participants and the person injecting the drug and placebo were blind to what drug they received or injected.

All patients underwent the usual surgical procedures, anesthesia, and analgesia. Approximately 1-2 hours before surgery, patients received oral gabapentin 600 mg, paracetamol in slow-release form 2 g, and celecoxib 400 mg. All patients undertook lumbar spinal anesthesia with 10 mg hyperbaric bupivacaine. Propofol (1-5 mg/kg/h) was ordered if necessary.
Primary outcomes included knee pain 4, 12, and 24 hours after surgery as assessed by a walker using a visual analog scale (VAS, 0 no pain and 100, worst imaginable pain). Similarly, pain at rest was assessed as a secondary outcome. Pain was evaluated 4, 12, and 24 hours after surgery [10]. Nausea was evaluated using a 4-point numerical scale (0: None, 1: Mild, 2: Moderate, 3: Severe) and the number of vomiting episodes [11]. In addition, patients’ performance indices after surgery were evaluated by the Western Ontario and McMaster universities osteoarthritis (WOMAC) index. The test questions are scored from 0 to 4 (0: None, 1: Mild, 2: Moderate, 3: Severe, and 4: Extreme). The scores for each subscale are summed up, with a possible score range of 0-68 for physical functions, 0-20 for pain, and 0-8 for stiffness [12].
To assess the knee’s range of motion, the subject lay comfortably on the examination bed, and the leg was placed flat. The axis of the goniometer was placed in the outer part of the knee on the tibial condyle, and the fixed arm was placed in the outer part of the thigh parallel to the longitudinal axis of the thigh. The movable arm was placed in the outer part of the leg parallel to the longitudinal axis of the tibia. The subject then flexed the knee, and the amount of flexion was measured. At the follow-up examination 6 weeks after the operation, the average pain during the day, in walking and at rest, was recorded [13]. In addition, the average degree of vomiting and nausea during the day, the quality of sleep at night, fatigue during the day, the daily intake of pain medication (type and dose), sleep medication (yes or no), anti-nausea medication (yes or no), side effects and infection were recorded. In addition, side effects, infections, and complications during hospitalization were recorded at the follow-up visit 6 weeks after the surgery. After collecting the information, all data were analyzed using SPSS software, version 16. The descriptive information was presented as Mean±SD or percentage. The Shapiro-Wilk test was used to assess the normality of the data. The t-test was used to compare quantitative variables in two groups with the assumption of normal distribution. A one-way test was used to compare quantitative variables in more than two groups. If the distribution of the variables was not normal, the non-parametric Mann-Whitney or Kruskal-Wallis tests were used. The paired t-test or its non-parametric equivalent (Wilcoxon signed-rank test) was used to compare the variables before and after the intervention. The chi-square test was used to compare qualitative variables. The repeated measures analysis of variance (ANOVA) was used to compare slightly repeated variables. P<0.05 were considered significant.
Results
In general, 75 patients (25 in each group) completed the study. Demographic characteristics in the control, Met, and Dex are summarized in Table 1. No significant difference was reported for any demographic variables in the three groups at the beginning of the study (P>0.05).

Table 2 compares the VAS scores in the three groups, showing significant differences at 4, 12, and 24 hours after the operation (P<0.001).

Figure 2 compares the mean score of the VAS in the three groups of study 4, 12, and 24 h after the operation.

Table 3 compares the WOMAC score between the Dex, Met, and placebo groups, which was not significantly different. Also, no significant difference was observed for WOMAC subscales in the three groups after treatment (P>0.05).

In terms of postoperative complications, the finding showed that postoperative nausea was reported in 16 patients (21.3%). The frequency of nausea for the placebo, Met, and Dex groups was 9(36%), 6(24%), and 1(4%), respectively, and the difference was statistically significant (P=0.002). No other complications were reported after the operation in the three groups. The result showed the range of motion was normal in all patients after surgery.
Discussion
In this study, we evaluated the effect of systemic corticosteroids on pain control after TKA. Reducing pain is the most important goal of knee joint replacement and is the demand of patients. Corticosteroids are applied to relieve inflammation of the joints and different body parts or as part of the treatment process for various diseases (such as severe allergies, skin problems, asthma, etc.) with two anti-inflammatory and immunosuppressive mechanisms in treating the disease. The risk of complications from the limited use of glucocorticoids in knee and hip arthroplasty surgery is not clearly defined [9]. Although multiple pain control methods and various surgical and anesthetic methods are used to manage these patients, pain is still one of the major problems of these patients after surgery [14, 15].
According to the results of the present study, most patients were female candidates for joint replacement surgery. The majority of patients underwent surgery in their sixth and seventh decades of life, so the mean age of these patients was 66. Most patients were overweight and obese, and their mean body mass index (BMI) was 28.07 kg/m2; obesity is associated with an increase in arthritis in the joints. Three treatment groups were completely homogeneous regarding demographic characteristics at the beginning of the study, and no significant difference was reported for any demographic variables in the three groups at the start of the study. The mean score of VAS in the three groups, 4, 12, and 24 hours after the operation, was significantly different in the treatment groups. In all time intervals evaluated after the operation, the pain intensity in the Dex user group was significantly lower than in the Met and placebo groups. Twenty-four hours after the operation, the mean pain score was significantly lower in the Dex and Met groups than in the placebo group. Also, the mean pain score 24 hours after the operation in the Dex group was significantly lower than in the Met group. No significant differences were observed for the mean WOMAC score and its subscales after surgery in the three groups. In other words, the use of corticosteroids after surgery was only related to pain control 24 hours after surgery.
Nausea was the most common complication after the operation and had the highest frequency in the placebo group and the lowest frequency in the Dex group, and this difference was statistically significant. In other words, Dex improved postoperative pain control and nausea. The results of our study are consistent with the other studies conducted in this field [16-21]. In line with the results of our research, in a clinical trial study, Kim et al. evaluated the effectiveness of systemic steroid use in reducing pain and nausea one day after TKA. They showed that the pain intensity in patients who received 0.1 mL Dex gram was significantly less than in the control group. Also, the severity of pain and nausea in another group of patients who received 0.2 mg/kg Dex 24 hours after surgery was lower than the control group and the group of patients who received 0.1 mg/kg Dex, which confirmed these results. In the study, similar to our study, no complications were reported in the groups. They showed that administering Dex before and after the operation was effective for controlling pain and nausea 24 hours after the operation [16], which is in line with the results of our study. In our study, the functional indicators, as well as the range of motion of the patients, were evaluated, and no significant difference was observed for these indicators in the groups, which shows that the administration of corticosteroids is only effective for reducing acute outcomes after surgery, such as pain control and reducing nausea.
In a meta-analysis, Liang et al. investigated the effect of intravenous Dex on postoperative pain control in patients undergoing TKA and showed that the addition of postoperative intravenous Dex for postoperative pain control in TKA significantly reduces analgesic use, the length of stay in the hospital and pain [17] and the systematic review confirmed our results. In the present research, the effect of Dex on reducing the length of hospital stay was not evaluated. Still, it is expected that with the decrease in pain intensity in patients, the length of hospital stay will also decrease in this group of patients. In another review paper, Liu et al. evaluated the effect of intravenous glucocorticoids before surgery in reducing acute pain after TKA, which examined 11 RCT studies, including 1000 patients (glucocorticoids=501, control=499). The results showed that preoperative intravenous glucocorticoids significantly reduced the VAS score for pain 6, 12, 24, and 48 hours after surgery. In addition, the use of these drugs was associated with a significant reduction of 19.4% and 16.8% in the incidence of nausea and vomiting, which was consistent with the results of our study. Our study shows intravenous glucocorticoids reduce postoperative pain and PONV in patients after TKA. However, more studies are needed to determine the maximum dose and type of glucocorticoids for better pain control in these patients [18]. Our study shows that administering Dex in lower doses, compared to other studies, effectively reduces pain in these patients without causing complications. In this regard, a study by Kardash et al. examined whether administering a single dose of Dex lowers dynamic pain after total hip arthroplasty in 50 patients. They reported that postoperative pain in the group Dex, as well as the need for painkillers and sedatives, decreased significantly [19]. In another study by Lunn et al., the effectiveness of Met 125 mg before surgery on pain and recovery after TKA was reported. It has a positive effect on pain control and recovery after TKA. Also, no side effects were observed in the study [20], which was in line with the results of our study. In our study, the use of Met was significantly associated with a reduction in postoperative pain and nausea compared to the control group. While the amount of pain reduction and nausea after surgery was lower for Met than for Dex. In another study, Backes et al. examined 120 patients who underwent knee and hip arthroplasty and reported that intravenous injection of 10 mg of Dex significantly reduced the prescription of anti-nausea and analgesic drugs and the duration of hospitalization compared to the control group [21]. In another study, Lex et al. concluded that intraoperative corticosteroids are safe, reduce postoperative pain, and accelerate patient recovery after unilateral TKA and total hip arthroplasty [22] in accordance with our study’s results.
Conclusion
This study shows that administering 24 mg of Dex and 125 mg of Met significantly reduces pain intensity and nausea 6, 12, and 24 hours after TKA. Met is significantly less effective than Dex in controlling pain and nausea. The administration of Dex and Met did not affect improvement based on the WOMAC index in patients after surgery. Given that nausea and pain persist for more than 24 hours after TKA, additional administration of Dex for a longer postoperative period should be considered. Considering the harmful effects of long-term use of these drugs with high doses, these results can be justified, and there is still a need to design and conduct clinical trial studies to determine the safe and effective dose of these drugs; it is felt that the present study is also the same.
Our study had some limitations that should be noted. The most important weakness of our study was the lack of estimation of some outcomes (such as duration of stay in hospital) due to the prevalence of the COVID-19 pandemic during the study. Moreover, with the spread of the COVID-19 pandemic, elective surgeries in medical centers decreased. The most important strength of the present study was the design of the study as a randomized controlled clinical trial with an appropriate sample size and the examination of the results in three intervention groups in parallel after the operation.
Ethical Considerations
Compliance with ethical guidelines
The present study followed the Declaration of Helsinki and the Ethical Guidelines for Medical and Health Research established by the Ministry of Health and Medical Education and the Ministry of Science, Research and Technology, Iran. This study obtained the approval by the Ethics Review Committee of Iran University of Medical Sciences, Tehran, Iran (Code: IR.IUMS.FMD.REC.1399.014). Informed consent was obtained from each patient included in the study.
Funding
The current research was funded by a specific project grant from the Iran University of Medical Sciences.
Authors' contributions
Conceptualization and supervision: Mehdi Moghtadaei, and Hossein Farahini; Methodology: Ali Yeganeh; Data collection and analysis: Sajad Amin Pirjeli; Investigation and writing: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to thank Iran University of Medical Sciences.
References
- Lau RL, Gandhi R, Mahomed S, Mahomed N. Patient satisfaction after total knee and hip arthroplasty. Clin Geriatr Med. 2012; 28(3):349-65. [DOI:10.1016/j.cger.2012.05.001]
- Zulkafli AW, Zakir NA, Othman AK, Mazelan A, Ismail R, Rahman WN, et al. The nursing approaches for pain management in post-operative total knee replacement. Asian J Med Biomed. 2022; 6(2):98-106. [DOI:10.37231/ajmb.2022.6.2.468]
- Fuzier R, Rousset J, Bataille B, Salces-y-Nédéo A, Maguès JP. One half of patients reports persistent pain three months after orthopaedic surgery. Anaesthesia Crit Care Pain Med. 2015; 34(3):159-64. [DOI:10.1016/j.accpm.2014.09.006]
- Vendittoli PA, Makinen P, Drolet P, Lavigne M, Fallaha M, Guertin MC, et al. A multimodal analgesia protocol for total knee arthroplasty: A randomized, controlled study. J Bone Joint Surg. 2006; 88(2):282-9. [DOI:10.2106/00004623-200602000-00005]
- McCracken G, Houston P, Lefebvre G. Guideline for the management of postoperative nausea and vomiting. J Obstet Gynaecol Can. 2008; 30(7):600-7. [DOI:10.1016/S1701-2163(16)32895-X]
- Kizilkaya M, Yildirim O, Ezirmik N, Kursad H, Karsan O. Comparisons of analgesic effects of different doses of morphine and morphine plus methylprednisolone after knee surgery. Eur J Anaesthesiol. 2005; 22(8):603-8. [DOI:10.1017/S0265021505001018]
- De Oliveira Jr GS, Castro-Alves LJS, Ahmad S, Kendall MC, McCarthy RJ. Dexamethasone to prevent postoperative nausea and vomiting: An updated meta-analysis of randomized controlled trials. Anesth Analg. 2013; 116(1):58-74. [DOI:10.1213/ANE.0b013e31826f0a0a]
- Waldron N, Jones C, Gan T, Allen T, Habib A. Impact of perioperative dexamethasone on postoperative analgesia and side-effects: Systematic review and meta-analysis. Br J Anaesth. 2013; 110(2):191-200. [DOI:10.1093/bja/aes431]
- Cutolo M, Seriolo B, Pizzorni C, Secchi ME, Soldano S, Paolino S, et al. Use of glucocorticoids and risk of infections. Autoimmun Rev. 2008; 8(2):153-5. [DOI:10.1016/j.autrev.2008.07.010]
- Fransen BL, Hoozemans MJ, Argelo KD, Keijser LC, Burger BJ. Fast-track total knee arthroplasty improved clinical and functional outcome in the first 7 days after surgery: A randomized controlled pilot study with 5-year follow-up. Arch Orthop Trauma Surg. 2018; 138:1305-16. [DOI:10.1007/s00402-018-3001-2]
- Ryu JH, Jeon YT, Min B, Hwang JY, Sohn HM. Effects of palonosetron for prophylaxis of postoperative nausea and vomiting in high-risk patients undergoing total knee arthroplasty: A prospective, randomized, double-blind, placebo-controlled study. Plos One. 2018; 13(5):e0196388. [DOI:10.1371/journal.pone.0196388]
- Lowry V, Ouellet P, Vendittoli PA, Carlesso LC, Wideman TH, Desmeules F. Determinants of pain, disability, health-related quality of life and physical performance in patients with knee osteoarthritis awaiting total joint arthroplasty. Disabil Rehabil. 2018; 40(23):2734-44. [DOI:10.1080/09638288.2017.1355412]
- Pua YH, Poon CLL, Seah FJT, Thumboo J, Clark RA, Tan M-H, et al. Predicting individual knee range of motion, knee pain, and walking limitation outcomes following total knee arthroplasty. Acta Orthop. 2019; 90(2):179-86. [DOI:10.1080/17453674.2018.1560647]
- Busch CA, Shore BJ, Bhandari R, Ganapathy S, MacDonald SJ, Bourne RB, et al. Efficacy of periarticular multimodal drug injection in total knee arthroplasty: A randomized trial. J Bone Joint Surg. 2006; 88(5):959-63. [DOI:10.2106/00004623-200605000-00005]
- Mullaji A, Kanna R, Shetty GM, Chavda V, Singh D. Efficacy of periarticular injection of bupivacaine, fentanyl, and methylprednisolone in total knee arthroplasty: A prospective, randomized trial. J Arthroplasty. 2010; 25(6):851-7. [DOI:10.1016/j.arth.2009.09.007]
- Kim JK, Lee HJ, Park JY, Han HS, Lee MC. Efficacy of systemic steroid use given one day after total knee arthroplasty for pain and nausea: A randomized controlled study. J Arthroplasty. 2020; 35(1):69-75. [DOI:10.1016/j.arth.2019.08.026]
- Liang S, Xing M, Jiang S, Zou W. Effect of intravenous dexamethasone on postoperative pain in patients undergoing total knee arthroplasty: A systematic review and meta-analysis. Pain Physician. 2022; 25(2):E169. [Link]
- Liu X, Liu J, Sun G. Preoperative intravenous glucocorticoids can reduce postoperative acute pain following total knee arthroplasty: A meta-analysis. Medicine. 2017; 96(35):e7836. [DOI:10.1097/MD.0000000000007836]
- Kardash KJ, Sarrazin F, Tessler MJ, Velly AM. Single-dose dexamethasone reduces dynamic pain after total hip arthroplasty. Anesth Analg. 2008; 106(4):1253-7. [DOI:10.1213/ANE.0b013e318164f319]
- Lunn T, Andersen LØ, Kristensen B, Husted H, Gaarn-Larsen L, Bandholm T, et al. Effect of high-dose preoperative methylprednisolone on recovery after total hip arthroplasty: A randomized, double-blind, placebo-controlled trial. Br J Anaesth. 2013; 110(1):66-73. [DOI:10.1093/bja/aes345]
- Backes JR, Bentley JC, Politi JR, Chambers BT. Dexamethasone reduces length of hospitalization and improves postoperative pain and nausea after total joint arthroplasty: A prospective, randomized controlled trial. J Arthroplasty. 2013; 28(8):11-7. [DOI:10.1016/j.arth.2013.05.041]
- Lex JR, Edwards TC, Packer TW, Jones GG, Ravi B. Perioperative systemic dexamethasone reduces length of stay in total joint arthroplasty: A systematic review and meta-analysis of randomized controlled trials. J Arthroplasty. 2021; 36(3):1168-86. [DOI:10.1016/j.arth.2020.10.010]
Type of Study: Research Article |
Subject:
Knee surgery
Received: 2022/11/2 | Accepted: 2022/12/12 | Published: 2023/02/1
Received: 2022/11/2 | Accepted: 2022/12/12 | Published: 2023/02/1
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |









