Volume 11, Issue 1 (2-2024)
JROS 2024, 11(1): 1-14 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Bagherifard A, Sahrai R, Mokhtari K, Yahyazedeh H. Therapeutic Impact of Platelet-rich Plasma on Anterior Cruciate Ligament Injury: A Mini-review. JROS 2024; 11 (1) :1-14
URL: http://jros.iums.ac.ir/article-1-2261-en.html
URL: http://jros.iums.ac.ir/article-1-2261-en.html
1- Department of Orthopedics, Bone and Joint Reconstruction Research Center, School of Medicine, Iran University of Medical Science, Tehran, Iran. & Department of Orthopedics, Shafayahyaeian Hospital, School of Medicine, Iran University of Medical Sciences,Tehran, Iran.
2- Department of Orthopedics, Bone and Joint Reconstruction Research Center, School of Medicine, Iran University of Medical Science, Tehran, Iran.
3- Department of Cell and Molecular Biology and Microbiology, Faculty of Biological Science and Technology, University of Isfahan, Isfahan, Iran.
4- Department of Orthopedics, Bone and Joint Reconstruction Research Center, School of Medicine, Iran University of Medical Science, Tehran, Iran. & Department of Orthopedic Surgery, Faculty of Medicine, Farhikhtegan Hospital, Tehran Medical Sciences Branch, Islamic Azad University, Tehran, Iran.
2- Department of Orthopedics, Bone and Joint Reconstruction Research Center, School of Medicine, Iran University of Medical Science, Tehran, Iran.
3- Department of Cell and Molecular Biology and Microbiology, Faculty of Biological Science and Technology, University of Isfahan, Isfahan, Iran.
4- Department of Orthopedics, Bone and Joint Reconstruction Research Center, School of Medicine, Iran University of Medical Science, Tehran, Iran. & Department of Orthopedic Surgery, Faculty of Medicine, Farhikhtegan Hospital, Tehran Medical Sciences Branch, Islamic Azad University, Tehran, Iran.
Keywords: Platelet-rich plasma (PRP), Anterior cruciate ligament (ACL), Tissue regeneration, Platelet dosage, Signaling pathway
Full-Text [PDF 1192 kb]
(314 Downloads)
| Abstract (HTML) (747 Views)
Full-Text: (327 Views)
Introduction
Platelet-rich plasma (PRP) offers a straightforward, effective, and minimally invasive approach to obtaining a natural concentration of autologous growth factors (GFs) [1]. PRP is obtained through the centrifugation of autologous blood, allowing for the separation and extraction of the plasma and buffy coat layers, which are enriched in platelets. PRP finds extensive application in multiple disciplines, such as dentistry, dermatology, plastic and maxillofacial surgery, acute trauma management, cosmetic surgery, and veterinary medicine [2-5]. The extensive application of PRP in the healing of various tissue types stems from its function as an easily accessible source of vital GFs and signaling molecules. These include catabolic cytokines derived from leukocytes and fibrinogen, which play critical roles in directing and regulating the tissue healing process [6]. This array of bioactive molecules facilitates a coordinated tissue-healing response to injury, progressing sequentially through the inflammatory, reparative, and remodeling phases of wound healing [7, 8]. On the other hand, PRP contains seven essential proteins, including platelet‐derived GF (PDGF), transforming GF-β (TGF-β), vascular endothelial GF (VEGF), epidermal GF (EGF), and adhesive proteins, such as fibrin, fibronectin and vitronectin [4, 9, 10].
GFs and their cellular origins and functional roles in tissue development and repair
GFs play crucial roles in regulating cell proliferation, differentiation, and migration, impacting tissue regeneration, immune responses, and cancer progression. Each GF originates from specific cell types and contributes distinct functions in various biological contexts. TGF-β is primarily secreted by platelets, neutrophils, and macrophages, and is fundamental in supporting the growth of undifferentiated mesenchymal cells. Additionally, TGF-β modulates endothelial cell function, promotes cartilage matrix formation, and influences natural killer cell activity in immune responses [11, 12, 13]. FGF, sourced from platelets, osteoblasts, and mesenchymal cells, acts as a potent mitogen for chondrocytes and osteoblasts, which are essential in bone formation and repair [11, 12, 13]. PDGF isoforms α and β, produced by platelets and osteoblasts, enhance cellular chemotaxis, thereby guiding cell movement to sites of tissue injury and repair [11, 12, 14]. EGF, secreted by platelets and osteoblasts, promotes mitosis in mesenchymal cells, fostering tissue regeneration and repair in wound healing contexts [11, 15, 16]. VEGF, derived from platelets and endothelial cells, is instrumental in angiogenesis, promoting endothelial cell proliferation and new blood vessel formation [11, 16, 17]. IGF, produced by platelets, macrophages, osteoblasts, and mesenchymal cells, stimulates mesenchymal cell mitogenesis and activates osteoblasts, thereby contributing to bone health and growth [11, 18, 19]. HGF, primarily sourced from platelets and mesenchymal cells, regulates cell growth and is crucial for tissue regeneration and repair processes [20]. KGF, originating from endothelial cells and fibroblasts, is essential in epithelial cell migration and proliferation, impacting wound closure and skin healing [20]. Ang-1, produced by platelets and neutrophils, plays a pivotal role in angiogenesis, enhancing blood vessel stability and integrity [20]. Platelet factor 4 (PF4), another platelet-derived GF, recruits leukocytes, aiding in immune response modulation at injury sites [20]. SDF-1α, produced by platelets, endothelial cells, and fibroblasts, is known for its ability to attract CD34+ stem cells, thereby supporting angiogenesis and tissue regeneration [20]. TNF, derived from neutrophils, T lymphocytes, and mast cells, is involved in regulating monocyte migration and fibroblast proliferation. These functions are critical for immune responses and inflammation control [20].
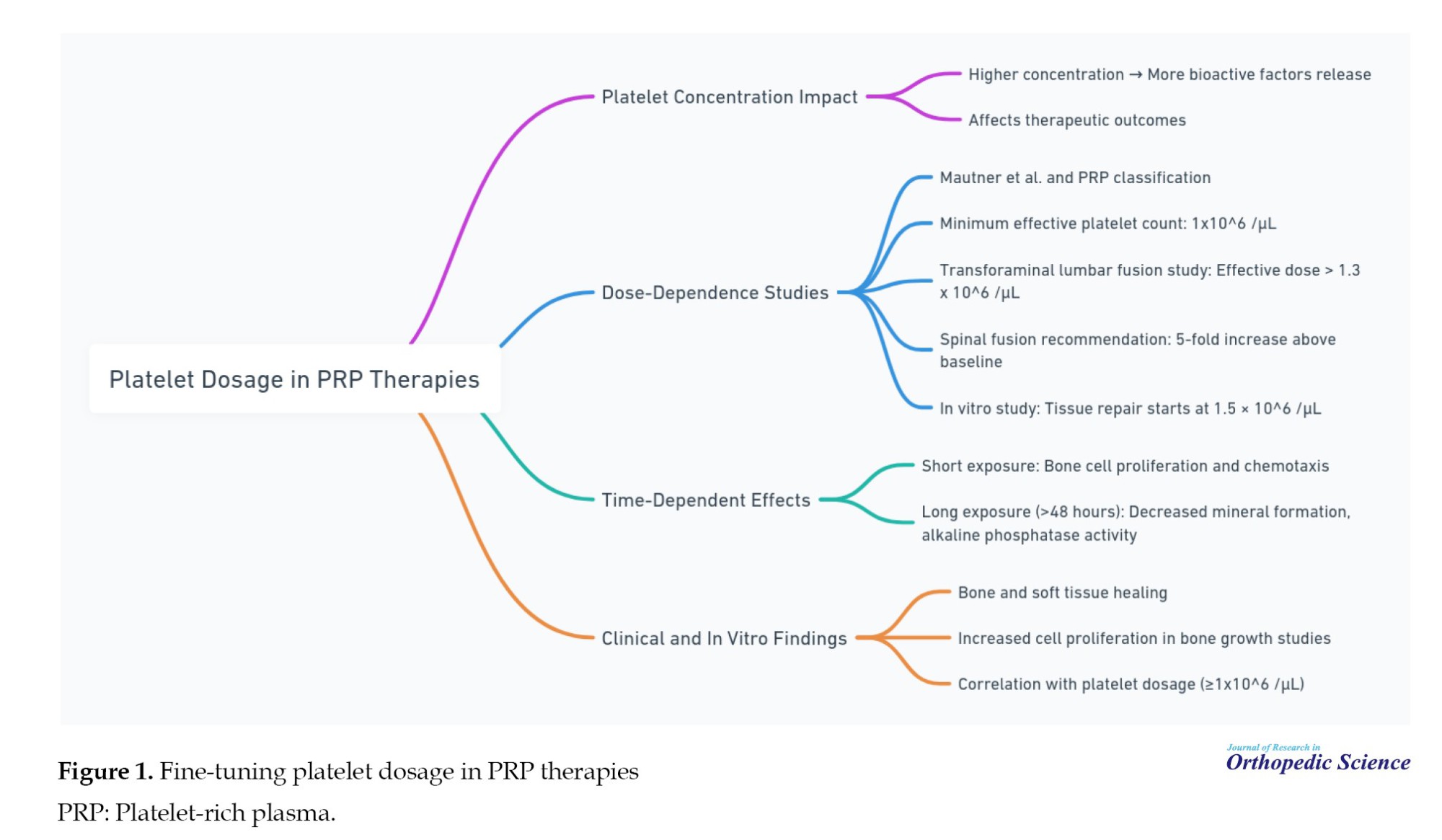
Fine-tuning platelet dosage in PRP therapies
Multiple dosage protocols for PRP therapies have been established, revealing dose-dependent effects on tissue healing and cellular responses. Elevated platelet concentrations in PRP formulations are anticipated to enhance the release of bioactive factors, thereby influencing therapeutic outcomes. Research indicates that cellular responses to PRP can vary based on platelet dosage, suggesting that an optimal concentration is crucial for effective tissue repair and angiogenesis. For example, studies recommend a minimum platelet concentration of 1×106/µL to facilitate healing in bone and soft tissues, with even higher doses associated with improved spinal fusion outcomes. Furthermore, the timing of PRP administration has been shown to affect treatment efficacy, as prolonged exposure may diminish the positive impact on cellular functions [21-28] (Figure 1).
Risk factors and contraindications
Current techniques for preparing PRP commonly utilize calcium and bovine thrombin to induce gel formation. Although the use of bovine thrombin has been associated with only a few reported cases of bleeding, it has been implicated in the production of antibodies against human coagulation factors V, XI, and thrombin, potentially resulting in serious coagulopathies. To address these concerns, alternative PRP activation agents have been introduced, such as autologous human thrombin, and synthetic compounds, like thrombin receptor agonist peptide-6 [29]. Autologous PRP therapy is generally considered safe for appropriately selected patients. Before initiating treatment, candidates should undergo a comprehensive hematological evaluation to rule out underlying coagulopathies or platelet dysfunction. Patients presenting with anemia or thrombocytopenia may be suboptimal candidates for PRP administration. Furthermore, contraindications may encompass clinical scenarios, such as hemodynamic instability, marked hypovolemia, active sepsis, unstable angina, and the concurrent use of anticoagulant or fibrinolytic agents.
Anterior cruciate ligament
The anterior cruciate ligament (ACL) is classified as an extrasynovial structure, and fibroblasts play a crucial role in its repair and continuous maintenance [30]. This ligament also functions to prevent excessive rotation of the tibia and to limit angulation in both varus and valgus directions [31]. ACL rupture is recognized as a prevalent injury associated with physical activity and is one of the leading causes of knee treatment requirements among young individuals [32, 33]. ACL rupture leads to diminished knee stability and can adversely affect a patient’s athletic performance. Additionally, it increases the likelihood of subsequent meniscus injuries and raises the risk of early-onset degeneration of the knee joint [34, 35].
Clinical use of PRP
The clinical use of PRP is based on its ability to enhance the concentration of GFs and stimulate protein secretion, thereby optimizing the healing process at the cellular level. PRP has been widely employed in the treatment of musculoskeletal injuries to facilitate recovery [36]. Despite its considerable potential for clinical application, the therapeutic use of PRP encounters challenges stemming from a lack of studies on technique standardization and inadequate descriptions of the procedures used. This highlights the urgent need to establish standardized criteria for producing high-quality PRP and to conduct further research to identify the optimal platelet concentration for various clinical scenarios. Clinical trials examining PRP for tendon injuries demonstrate significant variability in their approaches to preparation, quality control, dosage administration, and injection frequency. This inconsistency complicates the assessment of therapeutic efficacy. Additionally, differences in the types of cells involved, the release of inflammatory cytokines upon platelet activation, and the various methodologies used for PRP activation or non-activation further complicate the analysis [37]. The clinical outcomes associated with PRP treatments for chronic tendinopathy vary widely, ranging from significantly positive short- and long-term effects to positive outcomes that do not achieve statistical significance. This variability in results may be attributed to the differing stages of chronic tendinopathy, suggesting that PRP could be beneficial for certain stages while proving less effective for others (Table 1).
Anterior cruciate ligament reconstruction
In general, the management of ACL injuries can be categorized into non-surgical and surgical approaches. Physiotherapy plays a significant role in both treatment modalities—often enhancing the quality of care and expediting recovery, sometimes even prior to surgical intervention [54]. The primary objectives of physiotherapy include alleviating pain, inflammation, and swelling, enhancing the range of motion in the joint, strengthening muscles in accordance with the severity of the injury, conducting proprioceptive training, implementing closed-chain exercises, improving balance and functionality, and facilitating a quicker return to daily activities or competitive sports [55-57]. In elderly patients with an ACL rupture, surgical intervention is generally not advised due to their lower activity levels and the presence of knee osteoarthritis. However, surgery is typically recommended for younger individuals, particularly those who are active or professional athletes, in cases of complete ligament rupture [58, 59]. In patients who experience an injury to ACL along with a rupture of the medial collateral ligament (MCL), which is also quite common, the timing of ACL surgery is often postponed. This delay is implemented to mitigate the risk of arthrofibrosis [60, 61]. The management of complete ruptures can be approached through two surgical methods: i) Repair, which typically yields poor outcomes and is often unsuccessful, and ii) Reconstruction, which can be performed either intra-articularly or extra-articularly. During ligament reconstruction, an alternative tissue is utilized, such as the patellar tendon or the tendons of the hamstring muscles [62]. The reconstruction method generally yields superior outcomes. The primary objective of ACL reconstruction is to stabilize the knee joint. To accomplish this goal and reduce the complications associated with tendon resection, researchers have explored various techniques and approaches [63]. Various approaches, including autografts, allografts, and synthetic grafts, have been utilized for ACL reconstruction [64]. At present, autologous patellar tendon grafts, hamstring tendon grafts, and gracilis tendon grafts are the most frequently employed options for ACL reconstruction via an intra-articular approach. However, each of these methods is associated with potential side effects [65]. Also, PRP is an effective treatment modality for inflammatory conditions in orthopedics. PRP consists of autologous plasma enriched with a higher concentration of platelets compared to baseline levels, along with a variety of GFs that play a role in biosynthetic processes [66]. Administering concentrated PRP into the targeted area may activate various GFs that trigger regenerative processes in the context of acute injuries [67]. Despite the numerous studies on various methods of ACL reconstruction and their outcomes, a consensus regarding the superiority of one method or graft over others remains elusive. The debate continues over which graft type is optimal. Research indicates that PRP treatment may serve as a viable alternative to surgical intervention, enhancing patient performance and reducing disability [67, 68].
On the other hand, ACL reconstruction is generally regarded as a successful procedure, yielding excellent outcomes and high levels of patient satisfaction [69]. The maturation of the graft is crucial for achieving biomechanical strength and facilitating a return to activity. This process may be enhanced by the presence of GFs, such as PDGF, TGF-β1, and IGF-1 [69]. Research has demonstrated that PRP can enhance the viability and functionality of ACL cells, potentially accelerating the healing process of ACL grafts [70].
A prospective single-blind study involving 50 participants examined the effects of PRP on the recovery of patients who underwent ACL autograft surgery. The results indicated that those who received PRP gel during the procedure demonstrated significantly faster biological maturation as observed through MRI scans after one year (P<0.001) [69].
In another Level 1 study, the addition of platelet concentrate to both the semitendinosus graft and the femoral tunnel resulted in a significantly higher rate of graft maturation at the six-month mark, as evidenced by MRI scans [71].
In another study involving 30 patients, PRP was administered to the femoral tunnels; however, at the three-month mark, there were no significant differences in MRI findings related to graft maturation. This outcome may be a result of the shorter follow-up period and the smaller number of participants compared to previous studies [72].
A systematic review that analyzed eight trials found that the addition of PRP to ACL reconstruction could lead to an improvement in graft maturation of 20% to 30% [66].
Other studies have investigated the structural changes in the ACL graft following PRP administration. One such study examined 37 patients who underwent ACL reconstruction with hamstring grafts, comparing outcomes between those treated with PRP and those without. Second-look arthroscopies revealed that the patients who received PRP exhibited enhanced graft remodeling, characterized by connective tissue enveloping the graft and increased graft thickness. The researchers hypothesized that PRP may facilitate the “ligamentization” process in tendon grafts [73].
In contrast, a Level 1 randomized study involving 100 patients evaluated the effects of platelet-enriched gel in ACL reconstruction. At the two-year follow-up, no significant differences were found between the intervention and control groups in terms of functional or radiological outcomes. The authors suggested that this lack of observed benefit may have been influenced by multiple variables, such as the methods of PRP preparation and centrifugation, the type of graft utilized, variations in postoperative rehabilitation protocols, and the specific technique employed for PRP application [74]. Similar findings were reported by another research group, which observed no significant differences at the two-year follow-up following the use of PRP in ACL reconstruction with allografts [75].
PRP has been utilized at the harvest sites of patellar and tibial bone plugs. In a Level 1 study with 40 participants, the incorporation of PRP gel at these sites led to a significant enhancement in knee function at the one-year follow-up (P=0.041) [76].
In a separate study, 12 patients underwent PRP treatment for patellar tendon defects following the harvest for ACL reconstruction, while a control group of 15 patients did not receive PRP. Six-month MRI evaluations demonstrated that the patients who received PRP exhibited a significantly smaller gap in the patellar tendon compared to those in the control group [44].
In a study of 100 patients, those who underwent arthroscopic ACL tendon repair experienced significantly greater pain relief (mean VAS score of 3.05) and better range of motion (120.33º) compared to the PRP group (mean visual analogue scale [VAS] score of 4.39, range of motion 109.31º), with both differences being statistically significant (P=0.03) [77].
In a study on PRP therapy for ACL injuries, patients seeking an early return to sports received treatment within six weeks post-injury while wearing a brace that allowed weight bearing. MRI confirmed ligament continuity in all cases. The average patient age was 32.7 years, and they underwent an average of 2.8 PRP sessions. All patients returned to their pre-injury activity level (Tegner activity scale (TAS) 7.0) within approximately 139.5 days, with only one case of re-rupture reported. The results indicated that PRP therapy effectively restored ligament continuity and enabled a successful return to sports [78].
A systematic review of 14 randomized controlled trials examined the effects of PRP on ACL reconstruction (ACLR). The results showed that PRP injection improved pain (visual analog scale [VAS]) and knee function (International Knee Documentation Committee [IKDC]) significantly at three months post-surgery (P=0.0003 and P<0.00001, respectively). The pain was notably reduced in the first six months when PRP was applied at the bone–patellar tendon–bone harvest sites. However, long-term benefits were not observed, as PRP did not improve knee stability, prevent tunnel widening, or enhance graft healing. The study indicates that while PRP can provide short-term relief and functional improvement, further research is needed to understand its long-term impact [79].
A systematic review highlights the controversial clinical outcomes of using PRP for ACL augmentation. While the intraoperative application of PRP is considered safe and may reduce surgical morbidity by enhancing healing at the graft harvest site, its role in graft maturation is less favorable. Most studies indicate that PRP does not significantly improve graft integration, particularly in preventing bone tunnel widening. Additionally, PRP does not demonstrate superior clinical outcomes in the short term, and there is insufficient long-term data to evaluate its overall benefits in ACL surgery [80].
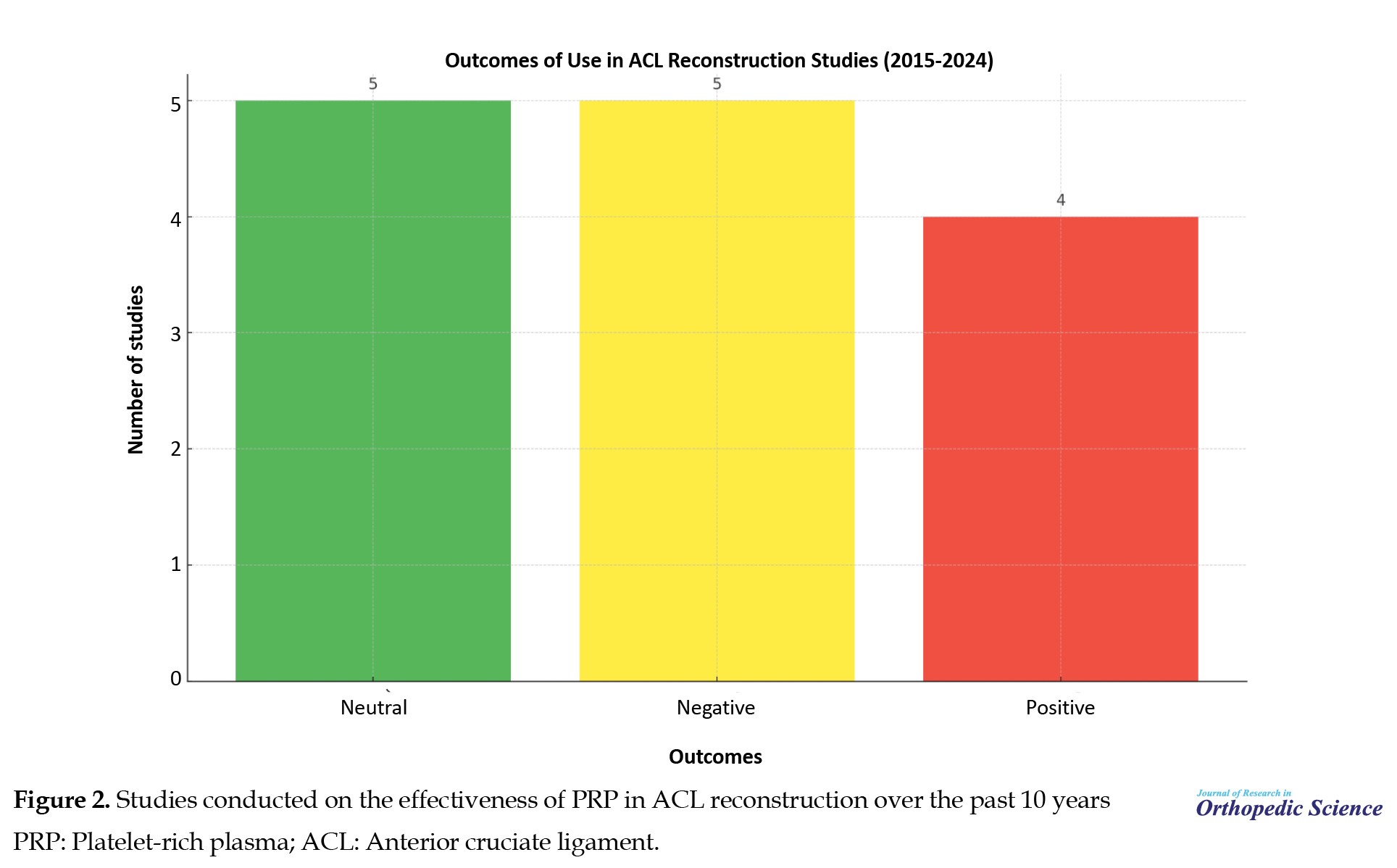
The outcomes of some of the most significant studies conducted on the effectiveness of PRP in ACL reconstruction over the past 10 years are presented in Table 2 and Figure 2.
PRP in osseous-tendinous junctions
The convergence of osseous and tendinous structures is critical in the context of ACL reconstruction, serving as a hallmark of successful surgical results [91]. While osseous integration represents the ideal healing process, the issue of donor site morbidity raises the need to explore effective alternatives [92]. A study has revealed a variety of promising alternatives, including innovative fixation substrates, osteogenic cytokines, hyperoxic therapies, and extracorporeal pulse activation techniques [93]. Among these options, PRP, enriched with GFs, stands out as a potential leader in promoting healing by enhancing cellular proliferation, matrix deposition, vasculogenesis, and collagen synthesis [93]. Several studies highlight the promising role of PRP in strengthening the integration of neo-ACLs; however, a consensus among orthopedic professionals is still lacking. Histomorphometric analyses indicate a positive trend, emphasizing improved bone-tendon integration in the presence of PRP [94]. Nonetheless, these studies had limitations, particularly the lack of biomechanical assessments and thorough evaluations of anchoring techniques. In contrast, a study involving lagomorphs presented a promising finding, demonstrating that PRP-enhanced tendon graft integration produced significantly better results compared to controls that did not receive PRP, which showed poorer integration [95]. In conclusion, PRP’s influence, ranging from rotator cuff repairs to ACL reconstructions, shows considerable promise. However, to definitively establish its superiority in orthopedic applications, there is a strong need for comprehensive and rigorous research.
Molecular mechanism
Previous studies have shown that PRP promotes ACL healing by increasing the expression of type I and type III collagen genes, enhancing the metabolism of ACL fibroblasts, and reducing cellular apoptosis [96, 97]. The blood supply to ACL originates from the small blood vessels of the middle genicular artery, as well as the subpatellar fat pad [98]. Following an ACL injury, a hypoxic microenvironment rapidly develops due to the rupture of blood vessels supplying the ligament [99, 100]. The low-oxygen microenvironment (3% oxygen concentration) negatively affects the proliferation and survival of ACL fibroblasts. This hypoxic condition is typically regarded as a catalyst for the accumulation of HIF-1α in ACL fibroblasts [101]. Numerous studies have shown that HIF-1α plays a role in the progression of cell apoptosis by enhancing the stability of p53 and promoting the overexpression of Bcl-2 [102, 103]. Elevated HIF-1α expression triggered apoptosis in ACL fibroblasts by upregulating pro-apoptotic proteins, including Bax and cleaved caspase-3, while downregulating anti-apoptotic proteins, such as Bcl-2 [101].
Trauma inevitably damages blood vessels, leading to an ischemic and hypoxic microenvironment following ACL injury [99]. Recently, with advancements in regenerative medicine, primary repair of the ACL has increasingly emerged as a viable treatment option for ACL injuries [104].
Fibroblasts responsible for forming the ACL comprised 60–70% of all cells within the ACL tissue. The survival and migration of these intrinsic ACL fibroblasts are regarded as essential prerequisites for effective ACL healing [105]. Effective ACL healing requires not only a sufficient blood supply but also the production of extracellular matrix and cellular proliferation [106].
ACL fibroblasts are abundant and synthesize ECM components, including collagen types I and III, which impart mechanical strength to the ACL tissue. Research indicates that PRP has a beneficial impact on ACL fibroblasts, significantly upregulating the gene expression of collagen types I and III and enhancing cell viability and metabolic activity [96]. They utilized 1% oxygen to create hypoxic conditions in human ligament-forming fibroblasts. A study demonstrated that these hypoxic environments resulted in a significant increase in the expression of collagen types I and III [107]. PRP may promote cell proliferation and migration in ACL fibroblasts, processes that necessitate significant ATP production. Furthermore, PRP has been shown to elevate the expression of VEGF in ACL tissue [108].
It was found that PRP significantly improved the migratory ability of ACL fibroblasts, especially at 72 hours following cell seeding [109]. GFs present in PRP, such as TGF-β and FGF, have been demonstrated to promote cell growth and migration [110, 111]. PDGF, a key bioactive constituent of PRP, interacts with PDGF receptors to initiate multiple intracellular signaling cascades, notably the MAPK and PI3K/Akt pathways [112]. These signaling pathways are essential regulators of cellular proliferation and apoptosis. Similarly, FGF-2 influences various cellular activities, particularly by promoting the proliferation and migration of ligament-derived cells [113, 114].
Both hypoxia and ATP depletion are necessary to trigger cell apoptosis. Sha et al. found that hypoxic conditions elevated HIF-1α expression, inducing apoptosis by upregulating pro-apoptotic proteins, such as cleaved caspase-3 and Bax while downregulating anti-apoptotic proteins, like Bcl-2 [101]. By utilizing an mTOR inhibitor, it was determined that HIF-1α functions as a downstream effector in the PI3K/Akt/mTOR signaling cascade [115].
A study revealed that PRP can safeguard ACL fibroblasts in hypoxic conditions by decreasing apoptosis and promoting cell viability, migration, and proliferation. However, PRP did not significantly influence the synthesis of collagen types I and III under hypoxia. The protective effects of PRP were attributed to the activation of the PI3K/Akt/mTOR signaling pathway [116].
Conclusion
Many studies on PRP have yielded varied and sometimes conflicting results regarding its effectiveness in ACL reconstruction, particularly concerning the optimal dosage and influencing factors. Recognizing these variations, this review underscores the importance of precise platelet dosing and other critical factors to maximize PRP’s efficacy. This analysis provides orthopedic surgeons with a comprehensive understanding of the controversies surrounding PRP application, aiding them in making evidence-based decisions for enhancing tissue repair and patient outcomes in ACL reconstruction. Well-powered clinical studies are essential for understanding PRP’s therapeutic effects fully.
Ethical Considerations
Compliance with ethical guidelines
There were no ethical considerations to be considered in this research.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Conceptualization and supervision: Hooman Yahyazedeh and Abolfazl Bagherifard; Methodology and data analysis: Reza Sahrai and Khatere Mokhtari; Data collection: Reza Sahrai and Abolfazl Bagherifard; Investigation and writing: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to express their gratitude to the Department of Orthopedics, School of Medicine, Bone and Joint Reconstruction Research Center, Shafayahyaeian Hospital, Iran University of Medical Sciences, Tehran, Iran, for their invaluable support in conducting this research. Additionally, the authors would like to thank the Department of Cellular and Molecular Biology and Microbiology, Faculty of Biological Science and Technology, University of Isfahan, Isfahan, Iran, for their assistance in providing the necessary expertise.
Reference
Platelet-rich plasma (PRP) offers a straightforward, effective, and minimally invasive approach to obtaining a natural concentration of autologous growth factors (GFs) [1]. PRP is obtained through the centrifugation of autologous blood, allowing for the separation and extraction of the plasma and buffy coat layers, which are enriched in platelets. PRP finds extensive application in multiple disciplines, such as dentistry, dermatology, plastic and maxillofacial surgery, acute trauma management, cosmetic surgery, and veterinary medicine [2-5]. The extensive application of PRP in the healing of various tissue types stems from its function as an easily accessible source of vital GFs and signaling molecules. These include catabolic cytokines derived from leukocytes and fibrinogen, which play critical roles in directing and regulating the tissue healing process [6]. This array of bioactive molecules facilitates a coordinated tissue-healing response to injury, progressing sequentially through the inflammatory, reparative, and remodeling phases of wound healing [7, 8]. On the other hand, PRP contains seven essential proteins, including platelet‐derived GF (PDGF), transforming GF-β (TGF-β), vascular endothelial GF (VEGF), epidermal GF (EGF), and adhesive proteins, such as fibrin, fibronectin and vitronectin [4, 9, 10].
GFs and their cellular origins and functional roles in tissue development and repair
GFs play crucial roles in regulating cell proliferation, differentiation, and migration, impacting tissue regeneration, immune responses, and cancer progression. Each GF originates from specific cell types and contributes distinct functions in various biological contexts. TGF-β is primarily secreted by platelets, neutrophils, and macrophages, and is fundamental in supporting the growth of undifferentiated mesenchymal cells. Additionally, TGF-β modulates endothelial cell function, promotes cartilage matrix formation, and influences natural killer cell activity in immune responses [11, 12, 13]. FGF, sourced from platelets, osteoblasts, and mesenchymal cells, acts as a potent mitogen for chondrocytes and osteoblasts, which are essential in bone formation and repair [11, 12, 13]. PDGF isoforms α and β, produced by platelets and osteoblasts, enhance cellular chemotaxis, thereby guiding cell movement to sites of tissue injury and repair [11, 12, 14]. EGF, secreted by platelets and osteoblasts, promotes mitosis in mesenchymal cells, fostering tissue regeneration and repair in wound healing contexts [11, 15, 16]. VEGF, derived from platelets and endothelial cells, is instrumental in angiogenesis, promoting endothelial cell proliferation and new blood vessel formation [11, 16, 17]. IGF, produced by platelets, macrophages, osteoblasts, and mesenchymal cells, stimulates mesenchymal cell mitogenesis and activates osteoblasts, thereby contributing to bone health and growth [11, 18, 19]. HGF, primarily sourced from platelets and mesenchymal cells, regulates cell growth and is crucial for tissue regeneration and repair processes [20]. KGF, originating from endothelial cells and fibroblasts, is essential in epithelial cell migration and proliferation, impacting wound closure and skin healing [20]. Ang-1, produced by platelets and neutrophils, plays a pivotal role in angiogenesis, enhancing blood vessel stability and integrity [20]. Platelet factor 4 (PF4), another platelet-derived GF, recruits leukocytes, aiding in immune response modulation at injury sites [20]. SDF-1α, produced by platelets, endothelial cells, and fibroblasts, is known for its ability to attract CD34+ stem cells, thereby supporting angiogenesis and tissue regeneration [20]. TNF, derived from neutrophils, T lymphocytes, and mast cells, is involved in regulating monocyte migration and fibroblast proliferation. These functions are critical for immune responses and inflammation control [20].
Fine-tuning platelet dosage in PRP therapies
Multiple dosage protocols for PRP therapies have been established, revealing dose-dependent effects on tissue healing and cellular responses. Elevated platelet concentrations in PRP formulations are anticipated to enhance the release of bioactive factors, thereby influencing therapeutic outcomes. Research indicates that cellular responses to PRP can vary based on platelet dosage, suggesting that an optimal concentration is crucial for effective tissue repair and angiogenesis. For example, studies recommend a minimum platelet concentration of 1×106/µL to facilitate healing in bone and soft tissues, with even higher doses associated with improved spinal fusion outcomes. Furthermore, the timing of PRP administration has been shown to affect treatment efficacy, as prolonged exposure may diminish the positive impact on cellular functions [21-28] (Figure 1).
Risk factors and contraindications
Current techniques for preparing PRP commonly utilize calcium and bovine thrombin to induce gel formation. Although the use of bovine thrombin has been associated with only a few reported cases of bleeding, it has been implicated in the production of antibodies against human coagulation factors V, XI, and thrombin, potentially resulting in serious coagulopathies. To address these concerns, alternative PRP activation agents have been introduced, such as autologous human thrombin, and synthetic compounds, like thrombin receptor agonist peptide-6 [29]. Autologous PRP therapy is generally considered safe for appropriately selected patients. Before initiating treatment, candidates should undergo a comprehensive hematological evaluation to rule out underlying coagulopathies or platelet dysfunction. Patients presenting with anemia or thrombocytopenia may be suboptimal candidates for PRP administration. Furthermore, contraindications may encompass clinical scenarios, such as hemodynamic instability, marked hypovolemia, active sepsis, unstable angina, and the concurrent use of anticoagulant or fibrinolytic agents.
Anterior cruciate ligament
The anterior cruciate ligament (ACL) is classified as an extrasynovial structure, and fibroblasts play a crucial role in its repair and continuous maintenance [30]. This ligament also functions to prevent excessive rotation of the tibia and to limit angulation in both varus and valgus directions [31]. ACL rupture is recognized as a prevalent injury associated with physical activity and is one of the leading causes of knee treatment requirements among young individuals [32, 33]. ACL rupture leads to diminished knee stability and can adversely affect a patient’s athletic performance. Additionally, it increases the likelihood of subsequent meniscus injuries and raises the risk of early-onset degeneration of the knee joint [34, 35].
Clinical use of PRP
The clinical use of PRP is based on its ability to enhance the concentration of GFs and stimulate protein secretion, thereby optimizing the healing process at the cellular level. PRP has been widely employed in the treatment of musculoskeletal injuries to facilitate recovery [36]. Despite its considerable potential for clinical application, the therapeutic use of PRP encounters challenges stemming from a lack of studies on technique standardization and inadequate descriptions of the procedures used. This highlights the urgent need to establish standardized criteria for producing high-quality PRP and to conduct further research to identify the optimal platelet concentration for various clinical scenarios. Clinical trials examining PRP for tendon injuries demonstrate significant variability in their approaches to preparation, quality control, dosage administration, and injection frequency. This inconsistency complicates the assessment of therapeutic efficacy. Additionally, differences in the types of cells involved, the release of inflammatory cytokines upon platelet activation, and the various methodologies used for PRP activation or non-activation further complicate the analysis [37]. The clinical outcomes associated with PRP treatments for chronic tendinopathy vary widely, ranging from significantly positive short- and long-term effects to positive outcomes that do not achieve statistical significance. This variability in results may be attributed to the differing stages of chronic tendinopathy, suggesting that PRP could be beneficial for certain stages while proving less effective for others (Table 1).

Anterior cruciate ligament reconstruction
In general, the management of ACL injuries can be categorized into non-surgical and surgical approaches. Physiotherapy plays a significant role in both treatment modalities—often enhancing the quality of care and expediting recovery, sometimes even prior to surgical intervention [54]. The primary objectives of physiotherapy include alleviating pain, inflammation, and swelling, enhancing the range of motion in the joint, strengthening muscles in accordance with the severity of the injury, conducting proprioceptive training, implementing closed-chain exercises, improving balance and functionality, and facilitating a quicker return to daily activities or competitive sports [55-57]. In elderly patients with an ACL rupture, surgical intervention is generally not advised due to their lower activity levels and the presence of knee osteoarthritis. However, surgery is typically recommended for younger individuals, particularly those who are active or professional athletes, in cases of complete ligament rupture [58, 59]. In patients who experience an injury to ACL along with a rupture of the medial collateral ligament (MCL), which is also quite common, the timing of ACL surgery is often postponed. This delay is implemented to mitigate the risk of arthrofibrosis [60, 61]. The management of complete ruptures can be approached through two surgical methods: i) Repair, which typically yields poor outcomes and is often unsuccessful, and ii) Reconstruction, which can be performed either intra-articularly or extra-articularly. During ligament reconstruction, an alternative tissue is utilized, such as the patellar tendon or the tendons of the hamstring muscles [62]. The reconstruction method generally yields superior outcomes. The primary objective of ACL reconstruction is to stabilize the knee joint. To accomplish this goal and reduce the complications associated with tendon resection, researchers have explored various techniques and approaches [63]. Various approaches, including autografts, allografts, and synthetic grafts, have been utilized for ACL reconstruction [64]. At present, autologous patellar tendon grafts, hamstring tendon grafts, and gracilis tendon grafts are the most frequently employed options for ACL reconstruction via an intra-articular approach. However, each of these methods is associated with potential side effects [65]. Also, PRP is an effective treatment modality for inflammatory conditions in orthopedics. PRP consists of autologous plasma enriched with a higher concentration of platelets compared to baseline levels, along with a variety of GFs that play a role in biosynthetic processes [66]. Administering concentrated PRP into the targeted area may activate various GFs that trigger regenerative processes in the context of acute injuries [67]. Despite the numerous studies on various methods of ACL reconstruction and their outcomes, a consensus regarding the superiority of one method or graft over others remains elusive. The debate continues over which graft type is optimal. Research indicates that PRP treatment may serve as a viable alternative to surgical intervention, enhancing patient performance and reducing disability [67, 68].
On the other hand, ACL reconstruction is generally regarded as a successful procedure, yielding excellent outcomes and high levels of patient satisfaction [69]. The maturation of the graft is crucial for achieving biomechanical strength and facilitating a return to activity. This process may be enhanced by the presence of GFs, such as PDGF, TGF-β1, and IGF-1 [69]. Research has demonstrated that PRP can enhance the viability and functionality of ACL cells, potentially accelerating the healing process of ACL grafts [70].
A prospective single-blind study involving 50 participants examined the effects of PRP on the recovery of patients who underwent ACL autograft surgery. The results indicated that those who received PRP gel during the procedure demonstrated significantly faster biological maturation as observed through MRI scans after one year (P<0.001) [69].
In another Level 1 study, the addition of platelet concentrate to both the semitendinosus graft and the femoral tunnel resulted in a significantly higher rate of graft maturation at the six-month mark, as evidenced by MRI scans [71].
In another study involving 30 patients, PRP was administered to the femoral tunnels; however, at the three-month mark, there were no significant differences in MRI findings related to graft maturation. This outcome may be a result of the shorter follow-up period and the smaller number of participants compared to previous studies [72].
A systematic review that analyzed eight trials found that the addition of PRP to ACL reconstruction could lead to an improvement in graft maturation of 20% to 30% [66].
Other studies have investigated the structural changes in the ACL graft following PRP administration. One such study examined 37 patients who underwent ACL reconstruction with hamstring grafts, comparing outcomes between those treated with PRP and those without. Second-look arthroscopies revealed that the patients who received PRP exhibited enhanced graft remodeling, characterized by connective tissue enveloping the graft and increased graft thickness. The researchers hypothesized that PRP may facilitate the “ligamentization” process in tendon grafts [73].
In contrast, a Level 1 randomized study involving 100 patients evaluated the effects of platelet-enriched gel in ACL reconstruction. At the two-year follow-up, no significant differences were found between the intervention and control groups in terms of functional or radiological outcomes. The authors suggested that this lack of observed benefit may have been influenced by multiple variables, such as the methods of PRP preparation and centrifugation, the type of graft utilized, variations in postoperative rehabilitation protocols, and the specific technique employed for PRP application [74]. Similar findings were reported by another research group, which observed no significant differences at the two-year follow-up following the use of PRP in ACL reconstruction with allografts [75].
PRP has been utilized at the harvest sites of patellar and tibial bone plugs. In a Level 1 study with 40 participants, the incorporation of PRP gel at these sites led to a significant enhancement in knee function at the one-year follow-up (P=0.041) [76].
In a separate study, 12 patients underwent PRP treatment for patellar tendon defects following the harvest for ACL reconstruction, while a control group of 15 patients did not receive PRP. Six-month MRI evaluations demonstrated that the patients who received PRP exhibited a significantly smaller gap in the patellar tendon compared to those in the control group [44].
In a study of 100 patients, those who underwent arthroscopic ACL tendon repair experienced significantly greater pain relief (mean VAS score of 3.05) and better range of motion (120.33º) compared to the PRP group (mean visual analogue scale [VAS] score of 4.39, range of motion 109.31º), with both differences being statistically significant (P=0.03) [77].
In a study on PRP therapy for ACL injuries, patients seeking an early return to sports received treatment within six weeks post-injury while wearing a brace that allowed weight bearing. MRI confirmed ligament continuity in all cases. The average patient age was 32.7 years, and they underwent an average of 2.8 PRP sessions. All patients returned to their pre-injury activity level (Tegner activity scale (TAS) 7.0) within approximately 139.5 days, with only one case of re-rupture reported. The results indicated that PRP therapy effectively restored ligament continuity and enabled a successful return to sports [78].
A systematic review of 14 randomized controlled trials examined the effects of PRP on ACL reconstruction (ACLR). The results showed that PRP injection improved pain (visual analog scale [VAS]) and knee function (International Knee Documentation Committee [IKDC]) significantly at three months post-surgery (P=0.0003 and P<0.00001, respectively). The pain was notably reduced in the first six months when PRP was applied at the bone–patellar tendon–bone harvest sites. However, long-term benefits were not observed, as PRP did not improve knee stability, prevent tunnel widening, or enhance graft healing. The study indicates that while PRP can provide short-term relief and functional improvement, further research is needed to understand its long-term impact [79].
A systematic review highlights the controversial clinical outcomes of using PRP for ACL augmentation. While the intraoperative application of PRP is considered safe and may reduce surgical morbidity by enhancing healing at the graft harvest site, its role in graft maturation is less favorable. Most studies indicate that PRP does not significantly improve graft integration, particularly in preventing bone tunnel widening. Additionally, PRP does not demonstrate superior clinical outcomes in the short term, and there is insufficient long-term data to evaluate its overall benefits in ACL surgery [80].
The outcomes of some of the most significant studies conducted on the effectiveness of PRP in ACL reconstruction over the past 10 years are presented in Table 2 and Figure 2.

PRP in osseous-tendinous junctions
The convergence of osseous and tendinous structures is critical in the context of ACL reconstruction, serving as a hallmark of successful surgical results [91]. While osseous integration represents the ideal healing process, the issue of donor site morbidity raises the need to explore effective alternatives [92]. A study has revealed a variety of promising alternatives, including innovative fixation substrates, osteogenic cytokines, hyperoxic therapies, and extracorporeal pulse activation techniques [93]. Among these options, PRP, enriched with GFs, stands out as a potential leader in promoting healing by enhancing cellular proliferation, matrix deposition, vasculogenesis, and collagen synthesis [93]. Several studies highlight the promising role of PRP in strengthening the integration of neo-ACLs; however, a consensus among orthopedic professionals is still lacking. Histomorphometric analyses indicate a positive trend, emphasizing improved bone-tendon integration in the presence of PRP [94]. Nonetheless, these studies had limitations, particularly the lack of biomechanical assessments and thorough evaluations of anchoring techniques. In contrast, a study involving lagomorphs presented a promising finding, demonstrating that PRP-enhanced tendon graft integration produced significantly better results compared to controls that did not receive PRP, which showed poorer integration [95]. In conclusion, PRP’s influence, ranging from rotator cuff repairs to ACL reconstructions, shows considerable promise. However, to definitively establish its superiority in orthopedic applications, there is a strong need for comprehensive and rigorous research.
Molecular mechanism
Previous studies have shown that PRP promotes ACL healing by increasing the expression of type I and type III collagen genes, enhancing the metabolism of ACL fibroblasts, and reducing cellular apoptosis [96, 97]. The blood supply to ACL originates from the small blood vessels of the middle genicular artery, as well as the subpatellar fat pad [98]. Following an ACL injury, a hypoxic microenvironment rapidly develops due to the rupture of blood vessels supplying the ligament [99, 100]. The low-oxygen microenvironment (3% oxygen concentration) negatively affects the proliferation and survival of ACL fibroblasts. This hypoxic condition is typically regarded as a catalyst for the accumulation of HIF-1α in ACL fibroblasts [101]. Numerous studies have shown that HIF-1α plays a role in the progression of cell apoptosis by enhancing the stability of p53 and promoting the overexpression of Bcl-2 [102, 103]. Elevated HIF-1α expression triggered apoptosis in ACL fibroblasts by upregulating pro-apoptotic proteins, including Bax and cleaved caspase-3, while downregulating anti-apoptotic proteins, such as Bcl-2 [101].
Trauma inevitably damages blood vessels, leading to an ischemic and hypoxic microenvironment following ACL injury [99]. Recently, with advancements in regenerative medicine, primary repair of the ACL has increasingly emerged as a viable treatment option for ACL injuries [104].
Fibroblasts responsible for forming the ACL comprised 60–70% of all cells within the ACL tissue. The survival and migration of these intrinsic ACL fibroblasts are regarded as essential prerequisites for effective ACL healing [105]. Effective ACL healing requires not only a sufficient blood supply but also the production of extracellular matrix and cellular proliferation [106].
ACL fibroblasts are abundant and synthesize ECM components, including collagen types I and III, which impart mechanical strength to the ACL tissue. Research indicates that PRP has a beneficial impact on ACL fibroblasts, significantly upregulating the gene expression of collagen types I and III and enhancing cell viability and metabolic activity [96]. They utilized 1% oxygen to create hypoxic conditions in human ligament-forming fibroblasts. A study demonstrated that these hypoxic environments resulted in a significant increase in the expression of collagen types I and III [107]. PRP may promote cell proliferation and migration in ACL fibroblasts, processes that necessitate significant ATP production. Furthermore, PRP has been shown to elevate the expression of VEGF in ACL tissue [108].
It was found that PRP significantly improved the migratory ability of ACL fibroblasts, especially at 72 hours following cell seeding [109]. GFs present in PRP, such as TGF-β and FGF, have been demonstrated to promote cell growth and migration [110, 111]. PDGF, a key bioactive constituent of PRP, interacts with PDGF receptors to initiate multiple intracellular signaling cascades, notably the MAPK and PI3K/Akt pathways [112]. These signaling pathways are essential regulators of cellular proliferation and apoptosis. Similarly, FGF-2 influences various cellular activities, particularly by promoting the proliferation and migration of ligament-derived cells [113, 114].
Both hypoxia and ATP depletion are necessary to trigger cell apoptosis. Sha et al. found that hypoxic conditions elevated HIF-1α expression, inducing apoptosis by upregulating pro-apoptotic proteins, such as cleaved caspase-3 and Bax while downregulating anti-apoptotic proteins, like Bcl-2 [101]. By utilizing an mTOR inhibitor, it was determined that HIF-1α functions as a downstream effector in the PI3K/Akt/mTOR signaling cascade [115].
A study revealed that PRP can safeguard ACL fibroblasts in hypoxic conditions by decreasing apoptosis and promoting cell viability, migration, and proliferation. However, PRP did not significantly influence the synthesis of collagen types I and III under hypoxia. The protective effects of PRP were attributed to the activation of the PI3K/Akt/mTOR signaling pathway [116].
Conclusion
Many studies on PRP have yielded varied and sometimes conflicting results regarding its effectiveness in ACL reconstruction, particularly concerning the optimal dosage and influencing factors. Recognizing these variations, this review underscores the importance of precise platelet dosing and other critical factors to maximize PRP’s efficacy. This analysis provides orthopedic surgeons with a comprehensive understanding of the controversies surrounding PRP application, aiding them in making evidence-based decisions for enhancing tissue repair and patient outcomes in ACL reconstruction. Well-powered clinical studies are essential for understanding PRP’s therapeutic effects fully.
Ethical Considerations
Compliance with ethical guidelines
There were no ethical considerations to be considered in this research.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Conceptualization and supervision: Hooman Yahyazedeh and Abolfazl Bagherifard; Methodology and data analysis: Reza Sahrai and Khatere Mokhtari; Data collection: Reza Sahrai and Abolfazl Bagherifard; Investigation and writing: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to express their gratitude to the Department of Orthopedics, School of Medicine, Bone and Joint Reconstruction Research Center, Shafayahyaeian Hospital, Iran University of Medical Sciences, Tehran, Iran, for their invaluable support in conducting this research. Additionally, the authors would like to thank the Department of Cellular and Molecular Biology and Microbiology, Faculty of Biological Science and Technology, University of Isfahan, Isfahan, Iran, for their assistance in providing the necessary expertise.
Reference
- Anand SX, Viles-Gonzalez JF, Badimon JJ, Cavusoglu E, Marmur JD. Membrane-associated CD40L and sCD40L in atherothrombotic disease. Thromb Haemost. 2003; 90(3):377-84. [DOI:10.1160/TH03-05-0268] [PMID]
- Cervelli V, Gentile P, Scioli MG, Grimaldi M, Casciani CU, Spagnoli LG, et al. Application of platelet-rich plasma in plastic surgery: Clinical and in vitro evaluation. Tissue Eng Part C Methods. 2009; 15(4):625-34. [DOI:10.1089/ten.tec.2008.0518] [PMID]
- Kazakos K, Lyras DN, Verettas D, Tilkeridis K, Tryfonidis M. The use of autologous PRP gel as an aid in the management of acute trauma wounds. Injury. 2009; 40(8):801-5. [DOI:10.1016/j.injury.2008.05.002] [PMID]
- Marx RE. Platelet-rich plasma: Evidence to support its use. J Oral Maxillofac Surg. 2004; 62(4):489-96. [DOI:10.1016/j.joms.2003.12.003] [PMID]
- Sánchez AR, Sheridan PJ, Kupp LI. Is platelet-rich plasma the perfect enhancement factor? A current review. Int J Oral Maxillofac Implants. 2003; 18(1):93-103. [PMID]
- Murphy G, Bretz U, Baggiolini M, Reynolds JJ. The latent collagenase and gelatinase of human polymorphonuclear neutrophil leucocytes. Biochem J. 1980; 192(2):517-25. [DOI:10.1042/bj1920517] [PMID]
- Velnar T, Bailey T, Smrkolj V. The wound healing process: an overview of the cellular and molecular mechanisms. J Int Med Res. 2009; 37(5):1528-42. [DOI:10.1177/147323000903700531] [PMID]
- Baniasadi M, Talebi S, Mokhtari K, Zabolian AH, Khosroshahi EM, Entezari M, et al. Role of non-coding RNAs in osteoporosis. Pathol Res Pract. 2024; 253:155036. [DOI:10.1016/j.prp.2023.155036] [PMID]
- Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983; 219(4587):983-5. [DOI:10.1126/science.6823562] [PMID]
- Herbst RS. Review of epidermal growth factor receptor biology. Int J Radiat Oncol Biol Phys. 2004; 59(2 Suppl):21-6. [DOI:10.1016/j.ijrobp.2003.11.041] [PMID]
- Raines EW, Ross R. Platelet-derived growth factor. I. High yield purification and evidence for multiple forms. J Biol Chem. 1982; 257(9):5154-60. [DOI:10.1016/S0021-9258(18)34649-0] [PMID]
- de Oliveira-Filho MA, Almeida LE, Pereira JA, Nunes Nassif PA, Czeczko NG, Kume MH, et al. Platelet-rich plasma in rabbits: Introduction of one experimental animal model. ABCD-Arquivos Brasileiros De Cirurgia Digestiva-Brazilian Archives of Digestive Surgery. 2008; 21(4):175-9. [DOI:10.1590/S0102-67202008000400005]
- Barnes GL, Kostenuik PJ, Gerstenfeld LC, Einhorn TA. Growth factor regulation of fracture repair. J Bone Miner Res. 1999; 14(11):1805-15. [DOI:10.1359/jbmr.1999.14.11.1805] [PMID]
- Wang JS. Basic fibroblast growth factor for stimulation of bone formation in osteoinductive and conductive implants. Acta Orthopaedica. 1996; 67(Supplement 272):133. [DOI:10.3109/17453679609158569]
- Friesel RE, Maciag T. Molecular mechanisms of angiogenesis: Fibroblast growth factor signal transduction. FASEB J. 1995; 9(10):919-25. [DOI:10.1096/fasebj.9.10.7542215] [PMID]
- Canalis E, McCarthy TL, Centrella M. Effects of platelet-derived growth factor on bone formation in vitro. J Cell Physiol. 1989; 140(3):530-7. [DOI:10.1002/jcp.1041400319] [PMID]
- Steenfos HH. Growth factors and wound healing. Scand J Plast Reconstr Surg Hand Surg. 1994; 28(2):95-105. [DOI:10.3109/02844319409071186] [PMID]
- Rhee JS, Black M, Schubert U, Fischer S, Morgenstern E, Hammes HP, et al. The functional role of blood platelet components in angiogenesis. Thromb Haemost. 2004; 92(2):394-402. [DOI:10.1160/TH03-04-0213] [PMID]
- Marx RE. Platelet-rich plasma: A source of multiple autologous growth factors for bone grafts. Berlin: Quintessence Publishing; 1999. [Link]
- Everts P, Onishi K, Jayaram P, Lana JF, Mautner K. Platelet-rich plasma: New performance understandings and therapeutic considerations in 2020. Int J Mol Sci. 2020; 21(20):7794. [DOI:10.3390/ijms21207794] [PMID]
- Mautner K, Malanga GA, Smith J, Shiple B, Ibrahim V, Sampson S, et al. A call for a standard classification system for future biologic research: The rationale for new PRP nomenclature. PM R. 2015; 7(4):S53-S9. [DOI:10.1016/j.pmrj.2015.02.005] [PMID]
- Nguyen PA, Pham TAV. Effects of platelet-rich plasma on human gingival fibroblast proliferation and migration in vitro. J Appl Oral Sci. 2018; 26:e20180077. [DOI:10.1590/1678-7757-2018-0077] [PMID]
- Graziani F, Ivanovski S, Cei S, Ducci F, Tonetti M, Gabriele M. The in vitro effect of different PRP concentrations on osteoblasts and fibroblasts. Clin Oral Implants Res. 2006; 17(2):212-9. [DOI:10.1111/j.1600-0501.2005.01203.x] [PMID]
- Hee HT, Majd ME, Holt RT, Myers L. Do autologous growth factors enhance transforaminal lumbar interbody fusion? Eur Spine J. 2003; 12(4):400-7. [DOI:10.1007/s00586-003-0548-5] [PMID]
- Park MS, Moon SH, Kim TH, Oh JK, Yoon WY, Chang HG. Platelet-rich plasma for the spinal fusion. J Orthop Surg. 2018; 26(1):2309499018755772. [DOI:10.1177/2309499018755772] [PMID]
- Giusti I, Rughetti A, D'Ascenzo S, Millimaggi D, Pavan A, Dell'Orso L, et al. Identification of an optimal concentration of platelet gel for promoting angiogenesis in human endothelial cells. Transfusion. 2009; 49(4):771-8. [DOI:10.1111/j.1537-2995.2008.02033.x] [PMID]
- Soffer E, Ouhayoun JP, Dosquet C, Meunier A, Anagnostou F. Effects of platelet lysates on select bone cell functions. Clin Oral Implants Res. 2004; 15(5):581-8. [DOI:10.1111/j.1600-0501.2004.01063.x] [PMID]
- Straum OK. The optimal platelet concentration in platelet-rich plasma for proliferation of human cells in vitro-diversity, biases, and possible basic experimental principles for further research in the field: A review. PeerJ. 2020; 8:e10303. [DOI:10.7717/peerj.10303] [PMID]
- Landesberg R, Burke A, Pinsky D, Katz R, Vo J, Eisig SB, et al. Activation of platelet-rich plasma using thrombin receptor agonist peptide. J Oral Maxillofac Surg. 2005; 63(4):529-35. [DOI:10.1016/j.joms.2004.12.007] [PMID]
- Raines BT, Naclerio E, Sherman SL. Management of anterior cruciate ligament injury: What's in and what's out? Indian J Orthop. 2017; 51(5):563-75. [DOI:10.4103/ortho.IJOrtho_245_17] [PMID]
- Werner BC, Yang S, Looney AM, Gwathmey FW Jr. Trends in pediatric and adolescent anterior cruciate ligament injury and reconstruction. J Pediatr Orthop. 2016; 36(5):447-52. [DOI:10.1097/BPO.0000000000000482] [PMID]
- Wetters N, Weber AE, Wuerz TH, Schub DL, Mandelbaum BR. Mechanism of injury and risk factors for anterior cruciate ligament injury. Operative Techniques in Sports Medicine. 2016; 24(1):2-6. [DOI:10.1053/j.otsm.2015.09.001]
- Andalib A, Etemadifar MR, Rafiee Zadeh A, Moshkdar P. Treatment of pilon fractures with low profile plates. Int J Burns Trauma. 2021; 11(6):486-93. [PMID]
- Parsons JL, Coen SE, Bekker S. Anterior cruciate ligament injury: towards a gendered environmental approach. Br J Sports Med. 2021; 55(17):984-90. [DOI:10.1136/bjsports-2020-103173] [PMID]
- Rafiee Zadeh A, Ghadimi K, Ataei A, Askari M, Sheikhinia N, Tavoosi N, et al. Mechanism and adverse effects of multiple sclerosis drugs: A review article. Part 2. Int J Physiol Pathophysiol Pharmacol. 2019; 11(4):105-114. [PMID]
- Marques LF, Stessuk T, Camargo IC, Sabeh Junior N, dos Santos L, Ribeiro-Paes JT. Platelet-rich plasma (PRP): Methodological aspects and clinical applications. Platelets. 2015; 26(2):101-13. [DOI:10.3109/09537104.2014.881991] [PMID]
- Kaux JF, Crielaard JM. Platelet-rich plasma application in the management of chronic tendinopathies. Acta Orthopaedica Belgica. 2013; 79(1):10-5. [Link]
- Castricini R, Longo UG, De Benedetto M, Panfoli N, Pirani P, Zini R, Maffulli N, et al. Platelet-rich plasma augmentation for arthroscopic rotator cuff repair: A randomized controlled trial. Am J Sports Med. 2011; 39(2):258-65. [DOI:10.1177/0363546510390780] [PMID]
- Volpi P, Marinoni L, Bait C, De Girolamo L, Schoenhuber H. Treatment of chronic patellar tendinosis with buffered platelet rich plasma: A preliminary study. Medicina Dello Sport. 2007; 60(4):595-603. [Link]
- Kon E, Filardo G, Delcogliano M, Presti ML, Russo A, Bondi A, et al. Platelet-rich plasma: New clinical application: A pilot study for treatment of jumper's knee. Injury. 2009; 40(6):598-603. [DOI:10.1016/j.injury.2008.11.026] [PMID]
- Kesikburun S, Tan AK, Yilmaz B, Yaşar E, Yazicioğlu K. Platelet-rich plasma injections in the treatment of chronic rotator cuff tendinopathy: A randomized controlled trial with 1-year follow-up. Am J Sports Med. 2013; 41(11):2609-16. [DOI:10.1177/0363546513496542] [PMID]
- Gotterbarm T, Richter W, Jung M, Berardi Vilei S, Mainil-Varlet P, Yamashita T, et al. An in vivo study of a growth-factor enhanced, cell free, two-layered collagen-tricalcium phosphate in deep osteochondral defects. Biomaterials. 2006; 27(18):3387-95. [DOI:10.1016/j.biomaterials.2006.01.041] [PMID]
- Filardo G, Kon E, Della Villa S, Vincentelli F, Fornasari PM, Marcacci M. Use of platelet-rich plasma for the treatment of refractory jumper's knee. Int Orthop. 2010; 34(6):909-15. [DOI:10.1007/s00264-009-0845-7] [PMID]
- de Almeida AM, Demange MK, Sobrado MF, Rodrigues MB, Pedrinelli A, Hernandez AJ. Patellar tendon healing with platelet-rich plasma: A prospective randomized controlled trial. Am J Sports Med. 2012; 40(6):1282-8. [DOI:10.1177/0363546512441344] [PMID]
- Filardo G, Kon E, Di Matteo B, Pelotti P, Di Martino A, Marcacci M. Platelet-rich plasma for the treatment of patellar tendinopathy: Clinical and imaging findings at medium-term follow-up. Int Orthop. 2013; 37(8):1583-9. [DOI:10.1007/s00264-013-1972-8] [PMID]
- de Vos RJ, Weir A, van Schie HT, Bierma-Zeinstra SM, Verhaar JA, Weinans H, et al. Platelet-rich plasma injection for chronic Achilles tendinopathy: A randomized controlled trial. JAMA. 2010; 303(2):144-9. [DOI:10.1001/jama.2009.1986] [PMID]
- Peerbooms JC, van Laar W, Faber F, Schuller HM, van der Hoeven H, Gosens T. Use of platelet rich plasma to treat plantar fasciitis: design of a multi centre randomized controlled trial. BMC Musculoskelet Disord. 2010; 11:69. [DOI:10.1186/1471-2474-11-69] [PMID]
- Owens RF Jr, Ginnetti J, Conti SF, Latona C. Clinical and magnetic resonance imaging outcomes following platelet rich plasma injection for chronic midsubstance Achilles tendinopathy. Foot Ankle Int. 2011; 32(11):1032-9. [DOI:10.3113/FAI.2011.1032] [PMID]
- Thanasas C, Papadimitriou G, Charalambidis C, Paraskevopoulos I, Papanikolaou A. Platelet-rich plasma versus autologous whole blood for the treatment of chronic lateral elbow epicondylitis: A randomized controlled clinical trial. Am J Sports Med. 2011; 39(10):2130-4. [DOI:10.1177/0363546511417113] [PMID]
- Randelli P, Arrigoni P, Ragone V, Aliprandi A, Cabitza P. Platelet rich plasma in arthroscopic rotator cuff repair: A prospective RCT study, 2-year follow-up. J Shoulder Elbow Surg. 2011; 20(4):518-28. [DOI:10.1016/j.jse.2011.02.008] [PMID]
- Mishra AK, Skrepnik NV, Edwards SG, Jones GL, Sampson S, Vermillion DA, et al. Efficacy of platelet-rich plasma for chronic tennis elbow: A double-blind, prospective, multicenter, randomized controlled trial of 230 patients. Am J Sports Med. 2014; 42(2):463-71. [DOI:10.1177/0363546513494359] [PMID]
- Rodeo SA, Delos D, Williams RJ, Adler RS, Pearle A, Warren RF. The effect of platelet-rich fibrin matrix on rotator cuff tendon healing: A prospective, randomized clinical study. Am J Sports Med. 2012; 40(6):1234-41. [DOI:10.1177/0363546512442924] [PMID]
- Gaweda K, Tarczynska M, Krzyzanowski W. Treatment of Achilles tendinopathy with platelet-rich plasma. Int J Sports Med. 2010; 31(8):577-83. [DOI:10.1055/s-0030-1255028] [PMID]
- Wahlstedt C, Rasmussen-Barr E. Anterior cruciate ligament injury and ankle dorsiflexion. Knee Surg Sports Traumatol Arthrosc. 2015; 23(11):3202-7. [DOI:10.1007/s00167-014-3123-1] [PMID]
- Rafiee Zadeh A, Ghadimi K, Mohammadi B, Hatamian H, Naghibi SN, Danaeiniya A. Effects of estrogen and progesterone on different immune cells related to multiple sclerosis. Caspian J Neurol Sci. 2018; 4(2):83-90. [DOI:10.29252/cjns.4.13.83]
- Hagino T, Ochiai S, Senga S, Yamashita T, Wako M, Ando T, et al. Meniscal tears associated with anterior cruciate ligament injury. Arch Orthop Trauma Surg. 2015; 135(12):1701-6. [DOI:10.1007/s00402-015-2309-4] [PMID]
- Cimino F, Volk BS, Setter D. Anterior cruciate ligament injury: diagnosis, management, and prevention. American family physician. 2010; 82(8):917-22. [Link]
- Rafiee Zadeh A, Farrokhi M, Etemadifar M, Beni AA. Prevalence of benign tumors among patients with multiple sclerosis. Am J Exp Clin Res. 2015; 2(4):127-32. [Link]
- Shen L, Jin ZG, Dong QR, Li LB. Anatomical risk factors of anterior cruciate ligament injury. Chin Med J. 2018; 131(24):2960-7. [DOI:10.4103/0366-6999.247207] [PMID]
- Rafiee Zadeh A, Falahatian M, Alsahebfosoul F. Serum levels of histamine and diamine oxidase in multiple sclerosis. Am J Clin Exp Immunol. 2018; 7(6):100-5. [PMID]
- Helito CP, Sobrado MF, Giglio PN, Bonadio MB, Pécora JR, Camanho GL, et al. Combined reconstruction of the anterolateral ligament in patients with anterior cruciate ligament injury and ligamentous hyperlaxity leads to better clinical stability and a lower failure rate than isolated anterior cruciate ligament reconstruction. Arthroscopy. 2019; 35(9):2648-54. [DOI:10.1016/j.arthro.2019.03.059] [PMID]
- Bayer S, Meredith SJ, Wilson KW, de Sa D, Pauyo T, Byrne K, et al. Knee morphological risk factors for anterior cruciate ligament injury: A systematic review. J Bone Joint Surg Am. 2020; 102(8):703-18. [DOI:10.2106/JBJS.19.00535] [PMID]
- Wellsandt E, Failla MJ, Snyder-Mackler L. Limb symmetry indexes can overestimate knee function after anterior cruciate ligament injury. J Orthop Sports Phys Ther. 2017; 47(5):334-8. [DOI:10.2519/jospt.2017.7285] [PMID]
- Duchman KR, Lynch TS, Spindler KP. Graft Selection in Anterior Cruciate Ligament Surgery: Who gets What and Why? Clin Sports Med. 2017; 36(1):25-33. [DOI:10.1016/j.csm.2016.08.013] [PMID]
- Kaeding CC, Pedroza AD, Reinke EK, Huston LJ, Hewett TE, Flanigan DC. Change in anterior cruciate ligament graft choice and outcomes over time. Arthroscopy. 2017; 33(11):200714. [DOI:10.1016/j.arthro.2017.06.019] [PMID]
- Figueroa D, Figueroa F, Calvo R, Vaisman A, Ahumada X, Arellano S. Platelet-rich plasma use in anterior cruciate ligament surgery: Systematic review of the literature. Arthroscopy. 2015; 31(5):981-8. [DOI:10.1016/j.arthro.2014.11.022] [PMID]
- Hutchinson ID, Rodeo SA, Perrone GS, Murray MM. Can platelet-rich plasma enhance anterior cruciate ligament and meniscal repair? J Knee Surg. 2015; 28(1):19-28. [DOI:10.1055/s-0034-1387166] [PMID]
- Everhart JS, Cavendish PA, Eikenberry A, Magnussen RA, Kaeding CC, Flanigan DC. Platelet-rich plasma reduces failure risk for isolated meniscal repairs but provides no benefit for meniscal repairs with anterior cruciate ligament reconstruction. Am J Sports Med. 2019; 47(8):1789-96. [DOI:10.1177/0363546519852616] [PMID]
- Radice F, Yánez R, Gutiérrez V, Rosales J, Pinedo M, Coda S. Comparison of magnetic resonance imaging findings in anterior cruciate ligament grafts with and without autologous platelet-derived growth factors. Arthroscopy. 2010; 26(1):50-7. [DOI:10.1016/j.arthro.2009.06.030] [PMID]
- Sánchez M, Anitua E, Lopez-Vidriero E, Andía I. The future: Optimizing the healing environment in anterior cruciate ligament reconstruction. Sports Med Arthrosc Rev. 2010; 18(1):48-53. [DOI:10.1097/JSA.0b013e3181c0ccd5] [PMID]
- Orrego M, Larrain C, Rosales J, Valenzuela L, Matas J, Durruty J, et al. Effects of platelet concentrate and a bone plug on the healing of hamstring tendons in a bone tunnel. Arthroscopy. 2008; 24(12):1373-80. [DOI:10.1016/j.arthro.2008.07.016] [PMID]
- Silva A, Sampaio R. Anatomic ACL reconstruction: does the platelet-rich plasma accelerate tendon healing? Knee Surg Sports Traumatol Arthrosc. 2009; 17(6):676-82. [DOI:10.1007/s00167-009-0762-8] [PMID]
- Sánchez M, Anitua E, Azofra J, Prado R, Muruzabal F, Andia I. Ligamentization of tendon grafts treated with an endogenous preparation rich in growth factors: Gross morphology and histology. Arthroscopy. 2010; 26(4):470-80. [DOI:10.1016/j.arthro.2009.08.019] [PMID]
- Nin JR, Gasque GM, Azcárate AV, Beola JD, Gonzalez MH. Has platelet-rich plasma any role in anterior cruciate ligament allograft healing? Arthroscopy. 2009; 25(11):1206-13. [DOI:10.1016/j.arthro.2009.06.002] [PMID]
- Magnussen RA, Flanigan DC, Pedroza AD, Heinlein KA, Kaeding CC. Platelet rich plasma use in allograft ACL reconstructions: Two-year clinical results of a MOON cohort study. Knee. 2013; 20(4):277-80. [DOI:10.1016/j.knee.2012.12.001] [PMID]
- Cervellin M, de Girolamo L, Bait C, Denti M, Volpi P. Autologous platelet-rich plasma gel to reduce donor-site morbidity after patellar tendon graft harvesting for anterior cruciate ligament reconstruction: A randomized, controlled clinical study. Knee Surg Sports Traumatol Arthrosc. 2012; 20(1):114-20. [DOI:10.1007/s00167-011-1570-5] [PMID]
- Eslami S, Fattah S, Taher SA, Rezasoltani Z. Platelet-rich plasma therapy or arthroscopic surgery on repair of anterior cruciate ligament rupture. Eur J Transl Myol. 2022; 32(3):10538. [DOI:10.4081/ejtm.2022.10538] [PMID]
- Hada S, Hada M, Yoshida K, Kaneko H, Saita Y, Kubota M, et al. Conservative Treatment Using Platelet-Rich Plasma for Acute Anterior Cruciate Ligament Injuries in Highly Active Patients: A Retrospective Survey. Cureus. 2024; 16(1):1-9. [DOI:10.7759/cureus.53102]
- Zhu T, Zhou J, Hwang J, Xu X. Effects of platelet-rich plasma on clinical outcomes after anterior cruciate ligament reconstruction: A systematic review and meta-analysis. Orthop J Sports Med. 2022; 10(1):23259671211061535. [DOI:10.1177/23259671211061535] [PMID]
- Andriolo L, Di Matteo B, Kon E, Filardo G, Venieri G, Marcacci M. PRP augmentation for ACL reconstruction. Biomed Res Int. 2015; 2015:371746. [DOI:10.1155/2015/371746] [PMID]
- Davey MS, Hurley ET, Withers D, Moran R, Moran CJ. Anterior cruciate ligament reconstruction with platelet-rich plasma: A systematic review of randomized control trials. Arthroscopy. 2020; 36(4):1204-10. [DOI:10.1016/j.arthro.2019.11.004] [PMID]
- Lv ZT, Zhang JM, Pang ZY, Wang Z, Huang JM, Zhu WT. The efficacy of platelet rich plasma on anterior cruciate ligament reconstruction: A systematic review and meta-analysis. Platelets. 2022; 33(2):229-41. [DOI:10.1080/09537104.2021.1902969] [PMID]
- Komzák M, Hart R, Šmíd P, Puskeiler M, Jajtner P. The effect of platelet-rich plasma on graft healing in reconstruction of the anterior cruciate ligament of the knee joint: prospective study. Acta Chir Orthop Traumatol Cech. 2015; 82(2):135-9. [DOI:10.55095/achot2015/019]
- Walters BL, Porter DA, Hobart SJ, Bedford BB, Hogan DE, McHugh MM, et al. Effect of intraoperative platelet-rich plasma treatment on postoperative donor site knee pain in patellar tendon autograft anterior cruciate ligament reconstruction: A double-blind randomized controlled trial. Am J Sports Med. 2018; 46(8):1827-35. [DOI:10.1177/0363546518769295] [PMID]
- Zicaro JP, Garcia-Mansilla I, Zuain A, Yacuzzi C, et al. Has platelet-rich plasma any role in partial tears of the anterior cruciate ligament? Prospective comparative study. World J Orthop. 2021; 12(6):423-432. [DOI:10.5312/wjo.v12.i6.423] [PMID]
- Utomo DN, Purwati P, Tinduh D, Wibowo NH. The effect of platelet rich plasma (PRP) in anterior cruciate ligament (ACL) reconstruction surgery. J Biomimetics Biomater Biomed Engin. 2017; 30:97-102. [DOI:10.4028/www.scientific.net/JBBBE.30.97]
- Zhang L, Zhang Q, Cui L, Wu L, Gao S. Kartogenin combined platelet-rich plasma (PRP) promoted tendon-bone healing for anterior cruciate ligament (ACL) reconstruction by suppressing inflammatory response via targeting AKT/PI3K/NF-κB. Appl Biochem Biotechnol. 2023; 195(2):1284-96. [DOI:10.1007/s12010-022-04178-y] [PMID]
- Ye Z, Chen H, Qiao Y, Wu C, Cho E, Wu X, et al. Intra-articular platelet-rich plasma injection after anterior cruciate ligament reconstruction: A randomized clinical trial. JAMA Netw Open. 2024; 7(5):e2410134. [DOI:10.1001/jamanetworkopen.2024.10134] [PMID]
- Zhang M, Zhen J, Zhang X, Yang Z, Zhang L, Hao D, et al. Effect of autologous platelet-rich plasma and gelatin sponge for tendon-to-bone healing after rabbit anterior cruciate ligament reconstruction. Arthroscopy. 2019; 35(5):1486-97. [DOI:10.1016/j.arthro.2018.11.014] [PMID]
- Cook JL, Smith PA, Bozynski CC, Kuroki K, Cook CR, Stoker AM, et al. Multiple injections of leukoreduced platelet rich plasma reduce pain and functional impairment in a canine model of ACL and meniscal deficiency. J Orthop Res. 2016; 34(4):607-15. [DOI:10.1002/jor.23054] [PMID]
- Kan H, Nakagawa S, Arai Y, Inoue A, Hino M, Komaki S, et al. Revision anterior cruciate ligament reconstruction using semitendinosus tendon with bone fragment. Arthrosc Tech. 2022; 11(12):e2397-401. [DOI:10.1016/j.eats.2022.08.047] [PMID]
- Garcia DC, Mingrone LE, de Sá MJC. Evaluation of osseointegration and bone healing using pure-phase β - TCP ceramic implant in bone critical defects. A systematic review. Front Vet Sci. 2022; 9:859920. [DOI:10.3389/fvets.2022.859920] [PMID]
- Branch EA, Matuska AM, Plummer HA, Harrison RM, Anz AW. Platelet-rich plasma devices can be used to isolate stem cells from synovial fluid at the point of care. Arthroscopy. 2021; 37(3):893-900. [DOI:10.1016/j.arthro.2020.09.035] [PMID]
- Zhang Y, Xing F, Luo R, Duan X. Platelet-rich plasma for bone fracture treatment: a systematic review of current evidence in preclinical and clinical studies. Front Med. 2021; 8:676033. [DOI:10.3389/fmed.2021.676033] [PMID]
- Ağır İ, Aytekin MN, Küçükdurmaz F, Kocaoğlu B, Çetinel S, Karahan M. The effect of platelet-rich plasma in bone-tendon integration. Adv Clin Exp Med. 2017; 26(2):193-9. [DOI:10.17219/acem/61384] [PMID]
- Cheng M, Wang H, Yoshida R, Murray MM. Platelets and plasma proteins are both required to stimulate collagen gene expression by anterior cruciate ligament cells in three-dimensional culture. Tissue Eng Part A. 2010; 16(5):1479-89. [DOI:10.1089/ten.tea.2009.0199] [PMID]
- Yoshida R, Cheng M, Murray MM. Increasing platelet concentration in platelet-rich plasma inhibits anterior cruciate ligament cell function in three-dimensional culture. J Orthop Res. 2014; 32(2):291-5. [DOI:10.1002/jor.22493] [PMID]
- Petersen W, Tillmann B. Structure and vascularization of the cruciate ligaments of the human knee joint. Anat Embryol. 1999; 200(3):325-34. [DOI:10.1007/s004290050283] [PMID]
- Hetsroni I, Manor A, Finsterbush A, Lowe J, Mann G, Palmanovich E. Reduced anterior cruciate ligament vascularization is associated with chondral knee lesions. Orthopedics. 2016; 39(4):e737-43. [DOI:10.3928/01477447-20160421-03] [PMID]
- Kittani M, Haviv B, Shemesh S, Yaari L, Yassin M, Rath-Wolfson L. Morphological and histological changes in the human anterior cruciate ligament after rupture. IMAJ. 2021; 23(1):33-7. [Link]
- Sha Y, Yang L, Lv Y. MGF E peptide improves anterior cruciate ligament repair by inhibiting hypoxia-induced cell apoptosis and accelerating angiogenesis. J Cell Physiol. 2019; 234(6):8846-61. [DOI:10.1002/jcp.27546] [PMID]
- Chen D, Li M, Luo J, Gu W. Direct interactions between HIF-1 alpha and Mdm2 modulate p53 function. J Biol Chem. 2003; 278(16):13595-8. [DOI:10.1074/jbc.C200694200] [PMID]
- Sowter HM, Ratcliffe PJ, Watson P, Greenberg AH, Harris AL. HIF-1-dependent regulation of hypoxic induction of the cell death factors BNIP3 and NIX in human tumors. Cancer Res. 2001; 61(18):6669-73. [Link]
- Murray MM, Kiapour AM, Kalish LA, Ecklund K; BEAR Trial Team; Freiberger C, et al. Predictors of healing ligament size and magnetic resonance signal intensity at 6 months after bridge-enhanced anterior cruciate ligament repair. Am J Sports Med. 2019; 47(6):1361-9. [DOI:10.1177/0363546519836087] [PMID]
- Naraoka T, Ishibashi Y, Tsuda E, Yamamoto Y, Kusumi T, Kakizaki I, et al. Time-dependent gene expression and immunohistochemical analysis of the injured anterior cruciate ligament. Bone Joint Res. 2012; 1(10):238-44. [DOI:10.1302/2046-3758.110.2000118] [PMID]
- Fermor B, Urban J, Murray D, Pocock A, Lim E, Francis M, et al. Proliferation and collagen synthesis of human anterior cruciate ligament cells in vitro: Effects of ascorbate-2-phosphate, dexamethasone and oxygen tension. Cell Biol Int. 1998; 22(9-10):635-40. [DOI:10.1006/cbir.1998.0302] [PMID]
- Kowalski TJ, Leong NL, Dar A, Wu L, Kabir N, Khan AZ, et al. Hypoxic culture conditions induce increased metabolic rate and collagen gene expression in ACL-derived cells. J Orthop Res. 2016; 34(6):985-94. [DOI:10.1002/jor.23116] [PMID]
- Li Y, Fu SC, Cheuk YC, Ong TY, Feng H, Yung SH. The effect of thermosensitive hydrogel platelet-rich-plasma complex in the treatment of partial tear of anterior cruciate ligament in rat model. J Orthop Translat. 2020 24:183-189. [DOI:10.1016/j.jot.2019.12.009] [PMID]
- Zheng H, Huang W, He B, Tan H, Lin P, Zha Z. Positive effects of platelet-rich plasma (PRP) and a Sanguisorba officinalis polysaccharide on the proliferation and differentiation of anterior cruciate ligament (ACL) fibroblasts in vitro. Pharm Biol. 2020; 58(1):297-305. [DOI:10.1080/13880209.2020.1743325] [PMID]
- Greenberger JS, Epperly MW, Jahroudi N, Pogue-Geile KL, Berry L, Bray J, et al. Role of bone marrow stromal cells in irradiation leukemogenesis. Acta Haematol. 1996; 96(1):1-15. [DOI:10.1159/000203708] [PMID]
- Everts PA, Knape JT, Weibrich G, Schönberger JP, Hoffmann J, Overdevest EP, et al. Platelet-rich plasma and platelet gel: A review. The Journal of extracorporeal technology. 2006; 38(2):174-87. [DOI:10.1051/ject/200638174]
- Papadopoulos N, Lennartsson J. The PDGF/PDGFR pathway as a drug target. Mol Aspects Med. 2018; 62:75-88. [DOI:10.1016/j.mam.2017.11.007] [PMID]
- Hankemeier S, Keus M, Zeichen J, Jagodzinski M, Barkhausen T, Bosch U, et al. Modulation of proliferation and differentiation of human bone marrow stromal cells by fibroblast growth factor 2: Potential implications for tissue engineering of tendons and ligaments. Tissue Eng. 2005; 11(1-2):41-9. [DOI:10.1089/ten.2005.11.41] [PMID]
- Takafuji H, Suzuki T, Okubo Y, Fujimura K, Bessho K. Regeneration of articular cartilage defects in the temporomandibular joint of rabbits by fibroblast growth factor-2: A pilot study. Int J Oral Maxillofac Surg. 2007; 36(10):934-7. [DOI:10.1016/j.ijom.2007.06.007] [PMID]
- Pore N, Jiang Z, Shu HK, Bernhard E, Kao GD, Maity A. Akt1 activation can augment hypoxia-inducible factor-1alpha expression by increasing protein translation through a mammalian target of rapamycin-independent pathway. Mol Cancer Res. 2006; 4(7):471-9. [DOI:10.1158/1541-7786.MCR-05-0234] [PMID]
- Cao Y, Li Y, Fu SC, Shen J, Zhang H, Jiang C, et al. Platelet-rich plasma pretreatment protects anterior cruciate ligament fibroblasts correlated with PI3K-Akt-mTOR pathway under hypoxia condition. J Orthop Translat. 2022; 34:102-112. [DOI:10.1016/j.jot.2022.02.002] [PMID]
Type of Study: Review Paper |
Subject:
Knee surgery
Received: 2023/11/26 | Accepted: 2023/12/28 | Published: 2024/02/1
Received: 2023/11/26 | Accepted: 2023/12/28 | Published: 2024/02/1
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |