Volume 10, Issue 4 (11-2023)
JROS 2023, 10(4): 183-200 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Pisoudeh K, Mokhtari K, Kazemi S. Recent Advanced in Imaging Technology for Diagnosing Hip Disorders: A Mini-review. JROS 2023; 10 (4) :183-200
URL: http://jros.iums.ac.ir/article-1-2262-en.html
URL: http://jros.iums.ac.ir/article-1-2262-en.html
1- Department of Orthopedics, Bone and Joint Reconstruction Research Center, School of Medicine, Shafayahyaeian Hospital, Iran University of Medical Sciences, Tehran, Iran
2- Department of Cell and Molecular Biology and Microbiology, Faculty of Biological Sciences and Technology, University of Isfahan, Isfahan, Iran.
3- Department of Orthopedics,Bone and Joint Reconstruction Research Center, School of Medicine, Iran University of Medical Sciences, Tehran, Iran. & Department of Orthopedics, School of Medicine, Imam Khomeini Hospital, Urmia University of Medical Sciences, Urmia, Iran.
2- Department of Cell and Molecular Biology and Microbiology, Faculty of Biological Sciences and Technology, University of Isfahan, Isfahan, Iran.
3- Department of Orthopedics,Bone and Joint Reconstruction Research Center, School of Medicine, Iran University of Medical Sciences, Tehran, Iran. & Department of Orthopedics, School of Medicine, Imam Khomeini Hospital, Urmia University of Medical Sciences, Urmia, Iran.
Keywords: Hip, Imaging, Artificial intelligence (AI), Deep learning, Magnetic resonance imaging (MRI), Computed tomography (CT), X-ray, Positron emission tomography (PET)/CT, PET/MRI, Nanotechnology, Three-dimensional (3D) imaging, Dynamic imaging, Low-dose imaging
Full-Text [PDF 1709 kb]
(111 Downloads)
| Abstract (HTML) (529 Views)
Full-Text: (128 Views)
Introduction
Hip disorders in medicine
Hip pain affects approximately 10% of the general population, and its prevalence progressively increases with age [1]. A published study revealed that 14.3% of adults experienced significant hip pain on most days within the preceding six weeks [2]. Hip pain is often associated with difficulty performing basic movements, such as sitting and standing, which can lead to chronic pain and adversely affect functional capacity and overall quality of life. Diagnosing hip pain can be complex due to potential referred pain from sources, such as the spine or knee, as well as conditions, such as trauma, tumors, abdominal or hernial issues, joint arthropathies, muscular disorders, and neuropathies [3].
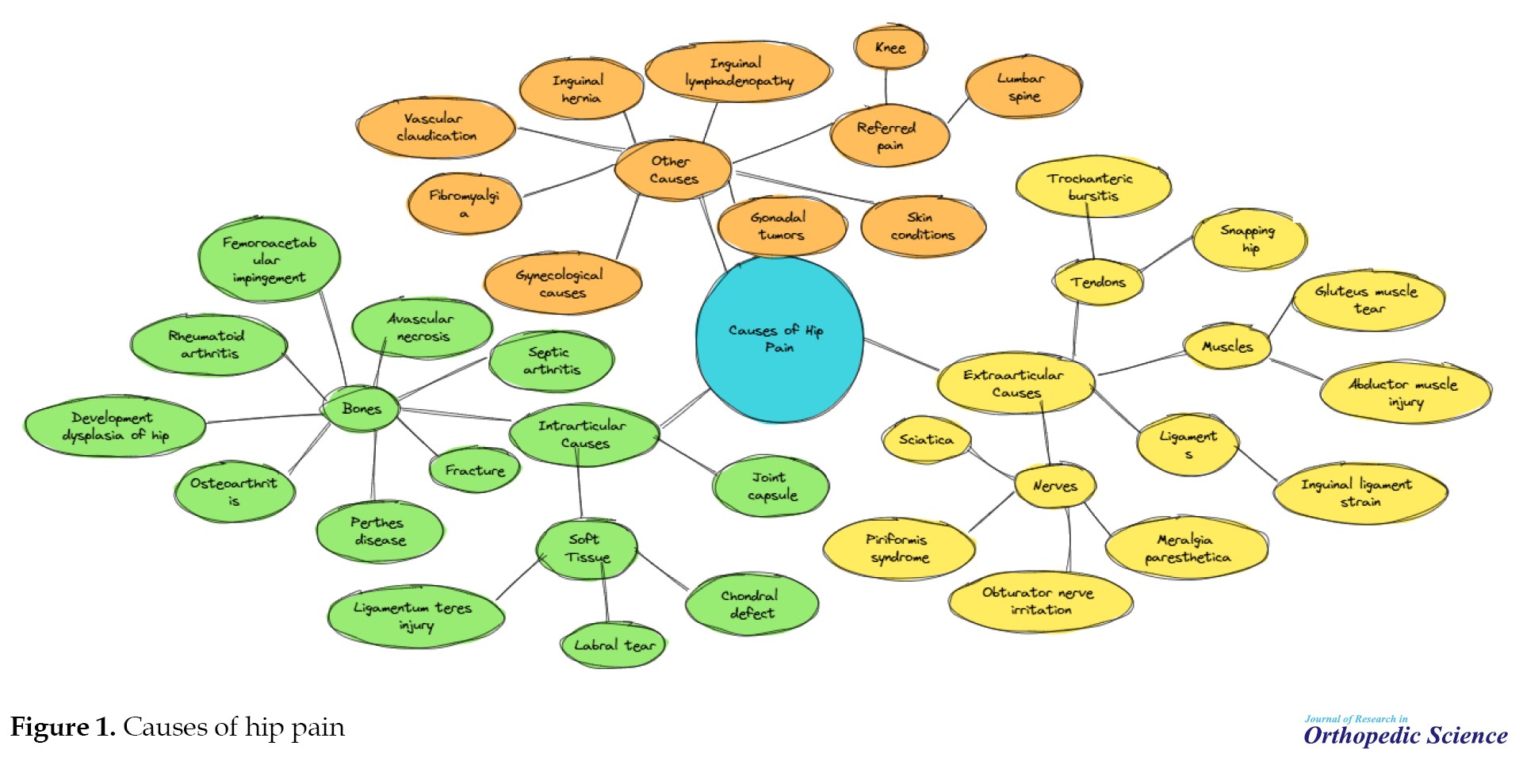
The hip joint, a ball-and-socket synovial joint, serves as a critical structure for weight transfer between the upper and lower body while facilitating movement across multiple planes. Although inherently shallow, the joint gains additional depth and stability from the labrum, a ring of fibrocartilage that encircles the acetabular rim [3]. The hyaline cartilage envelops the articular surfaces of the hip joint and efficiently absorbs and dissipates shear and compressive forces during movement. The joint’s structural integrity is reinforced by ligaments, with the iliofemoral and pubofemoral ligaments providing anterior support and the ischiofemoral ligament stabilizing posteriorly [4]. The hip joint is encircled by numerous muscle groups that facilitate a broad range of movements and contribute to versatility and functional mobility [4]. The trochanteric, iliopsoas, gluteus medius, and ischiogluteal bursae serve as cushions between the bones and surrounding tendons of the hip joint, reducing friction and facilitating smooth movement. The hip joint receives its nerve supply from the articular branches of the quadratus femoris nerve, obturator nerve, femoral nerve, sciatic nerve, and nerves supplying nearby muscles, including the superior and inferior gluteal nerves [5]. Multiple nerves’ complex innervation of the hip joint makes it challenging to differentiate primary hip pain from radicular pain originating from the lumbar spine [3] (Figure 1).
The role of Imaging technologies in improving diagnosis
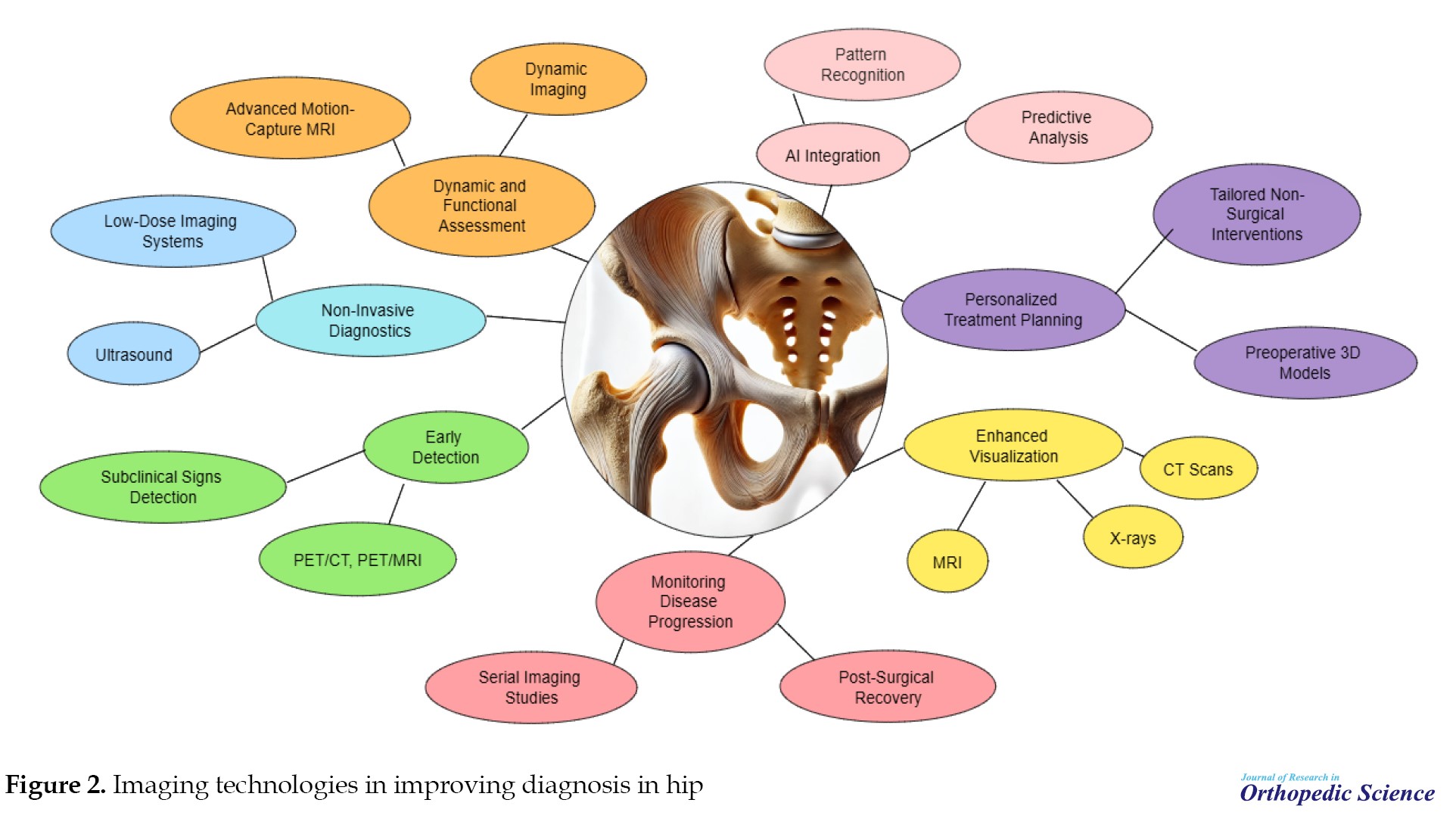
Imaging technologies play a pivotal role in diagnosing, assessing, and managing hip disorders by providing detailed insights into the anatomical, functional, and metabolic aspects of the hip joint. These technologies significantly enhance clinicians’ ability to detect abnormalities, monitor disease progression, and plan effective treatments [6, 7, 8]. Key ways imaging technologies contribute to improved diagnosis (Figure 2). This mini-review aims to provide an overview of recent advancements in imaging technologies and their applications in diagnosing hip disorders. Since hip pathologies significantly impact patient mobility and quality of life, timely and accurate diagnosis is crucial for effective management. This article examines how innovations in imaging techniques, from traditional modalities to cutting-edge technologies, have enhanced the detection, evaluation, and treatment planning for hip disorders.
Conventional imaging technologies for diagnosing hip disorders
X-ray imaging
Despite significant advancements in three-dimensional (3D) medical imaging technologies, planar x-ray imaging continues to be widely utilized in routine clinical practice. Traditional x-ray devices are relatively cost-effective and facilitate convenient image acquisition. However, accurate interpretation of x-ray radiographs remains challenging. This is primarily due to the projection of 3D anatomical structures onto a 2D plane, resulting in a loss of depth perception. Additionally, the radiographic magnification factor is typically approximated. Although newer generations of x-ray devices can standardize image acquisition, accurately estimating the magnification remains a significant challenge. X-ray radiographs are widely used in clinical settings for various purposes, including diagnostic assessment, pre-operative planning, and post-operative evaluation. Despite advancements in imaging technology, magnification distortion still requires careful consideration when interpreting radiographs for precise measurements and treatment decisions. Various methods have been proposed to enhance the accuracy [9-11]. A coin placed on the patient’s skin during x-ray acquisition was utilized as the reference object [9]. A prospective study demonstrated that the correct hip prosthesis size estimation can be improved from 59.4% to 68.8% when using a coin as a reference object, compared to using a caliper [10]. They showed that the use of a coin as a reference object allowed for a more accurate estimation of the radiographic magnification. They proposed a method utilizing a spherical object, which is adjustable in height and must be placed between the patient’s legs during imaging [11-14]. They employed a precise calibration method alongside the triangulation of manually identified points to reconstruct the spine and rib cage from biplanar x-ray images [13].
Computed tomography (CT) scans
In CT scanning, a series of x-ray beams and detectors are arranged in a circular configuration around the patient, who is positioned at the center of this circle. The x-ray beam passes through the patient with either the beam or detector rotating, generating complex images. These images are then mathematically reconstructed using a computer, enabling high-quality CT scan details. The data collected by the detectors are stored on tape and processed by a computer to create a cross-sectional image [15, 16]. This method differs significantly from the standard frontal and sagittal planes produced using traditional x-ray films, where the beam passes through the patient to a plate on the opposite side. Each structure along the beam’s path is captured in CT scanning, with denser structures masking those of lower densities. The cross-sectional images can be adjusted in thickness, with modern machines capable of producing slices as thin as 0.5 cm. These thin slices help eliminate image overlap, offering a clearer representation of the structures being defined. Once the image is captured, mathematical reconstructions allow for adjustments in image density using data stored on a computer tape. The operator can enhance structures of interest and exclude others, enabling selective examination of soft tissues, fluid collections, bone, or air densities without additional patient exposure. This capability is particularly valuable for assessing the musculoskeletal system, where bony structures are centrally located and surrounded by soft tissues. The complex three-dimensional anatomy of structures, such as the pelvis or spine, complicates the evaluation of bone and intra-articular injuries [17, 18]. Many musculoskeletal soft-tissue tumors, such as sarcomas, have tissue densities that closely resemble the surrounding tissues, making them undetectable on standard x-ray films. In these cases, alternative techniques, such as arteriography and radiography have been developed to help identify them, albeit with limited success. CT scanning addresses several of these limitations. However, CT scanning has some drawbacks. First, current models are less capable of distinguishing between two closely spaced points compared to routine x-ray films, although this issue is being gradually improved with further advancements. Second, the radiation dose from a CT scan is relatively high, ranging from one to two rads per slice or eight-ten rads per study. This is a significant dose that should be considered when ordering the procedure. The radiation dose increases when finer detail is needed but can be reduced if image quality is less critical (for example, in studies focusing on bone density). Finally, CT scans are expensive. Despite the high cost of machines (often approaching $750000), their extensive use in fields, such as medicine, surgery, neurology, and neurosurgery has proven to be cost-effective when applied appropriately. Due to their increasing utilization, the cost per study has significantly decreased over the years, and regional access has further helped reduce costs [19, 20]. Despite these limitations, CT scanners have become essential tools in specific orthopedic situations and should be considered in diagnosing musculoskeletal disorders [21, 22]. Radiographic evaluation of trauma to the femoral head and acetabulum traditionally involves anteroposterior and lateral x-rays, oblique views, and tomography. However, these methods can be hindered by the significant overlap of structures anterior and posterior to the hip, requiring correlation of data from various views to understand the fracture’s anatomy. As noted by Epstein, loose fragments within the joint can affect functional outcomes and should be removed if present. Cross-sectional imaging provided by CT scanning proved highly effective in visualizing the third dimension of hip trauma. One of the key advantages of CT scanning is its ability to detect subtle density changes, which helps identify intraarticular fragments, minor fractures, and dome fractures that may not be visible on routine x-rays or tomograms. The CT scan provides a clear view of the superior margin of the acetabulum, pelvic wall medial to the acetabulum and femoral head, and femoral head and neck because sections are obtained serially from cranial to caudal. This allows for precise assessment of the relationships between these structures, such as medial migration of the femoral head following a fracture of the central acetabulum. Since CT scans are performed with the patient in a supine position, traction can be maintained during the procedure, enabling the assessment of the effectiveness of traction in reducing the fracture. Moreover, the absence of oblique positioning makes CT scanning more comfortable for patients than oblique positioning. Additionally, complex fractures of the extraarticular portion of the pelvic bones, including sacroiliac joint disruptions, can be effectively evaluated using CT [21, 23].
Magnetic resonance imaging (MRI)
On MRI, the normal femoral head in adults typically demonstrates homogeneous high signal intensity on T1-weighted sequences, a characteristic attributed to its fatty marrow content. In contrast, the femoral neck and intertrochanteric region showed lower signal intensity on T1-weighted images due to red marrow and a reduced amount of fatty marrow. Articular hyaline cartilage, composed of a complex mixture of water, collagen, and proteoglycans, is crucial for distributing forces, absorbing pressure, and facilitating smooth gliding of the bony structures within the joint, thereby ensuring optimal joint function and mobility [24]. The entire femoral head is encased in articular hyaline cartilage, except a small area at the fovea that lacks cartilage. On MRI, normal articular hyaline cartilage typically presents with intermediate to high signal intensity on fluid-sensitive sequences, reflecting its water content. This characteristic indicates its crucial role in joint function, as cartilage facilitates smooth movement, reduces friction, and helps distribute mechanical loads across the joint surfaces [25, 26].
Although the normal acetabular labrum is typically hypointense and triangular, there is variability in both its morphological appearance and signal intensity among asymptomatic individuals. This variability can make interpretation more challenging because the labrum may present slight differences in shape, size, and signal characteristics in healthy individuals [27]. Intermediate-to-high signal intensity within the labral substance on MRI may result from small intralabral fibrovascular bundles. Additionally, increased signal intensity in the labrum on T1-weighted images can be attributed to the “magic angle” effect, which occurs when structures with a collagen-rich composition, such as the labrum, are imaged at a specific angle relative to the magnetic field, causing an increase in signal intensity [28]. A normal, mildly hyperintense 1-2 mm transition zone can be observed at the chondrolabral junction on MRI. This zone represents the interface between the articular hyaline cartilage and labrum, where there is typically a gradual change in signal intensity due to differences in tissue composition, such as the transition from cartilage to fibrocartilage [24]. Normal cartilage can “undercut” the labrum at the chondrolabral junction, which appears as a smooth focus of intermediate-to-high signal intensity on MRI. This area isointense to the cartilage and is situated between the labrum and acetabular rim. It represents a natural anatomical feature where the cartilage slightly extends beneath the labrum, contributing to joint stability and function [26]. A sublabral sulcus is a fluid-filled cleft located at the chondrolabral junction and is typically found anteroinferiorly at the four o’clock position. It is considered a normal variant in many cases. Additionally, a normal labro-ligamentous sulcus may be observed at the junction between the labrum and transverse acetabular ligament. As individuals age, the labrum may undergo degenerative changes, resulting in blunting, signal alterations, or complete absence of the labrum in some instances [29].
Magnetic resonance arthrography (MRA) is the current gold standard for assessing the acetabular labrum and hip hyaline articular cartilage. This technique involves the injection of contrast material into the joint, which helps separate the internal structures, allowing for clear visualization of the labrum and cartilage. The contrast extends into labral tears or chondral defects, making it highly effective for detecting these abnormalities and providing detailed images of the joint’s soft tissues [28, 30]. MRA offers higher diagnostic accuracy than non-contrast MRI for detecting acetabular cartilage lesions, whether performed at 1.5T or 3T. Using contrast material in MRA enhances the differentiation of cartilage defects and labral tears, providing more detailed and clearer images than standard non-contrast MRI, which improves the overall detection of joint abnormalities [31]. Despite the use of intra-articular contrast in MRA, the cartilages of the acetabulum and femoral head are often not delineated, making it challenging to visualize small lesions. However, hip MRA performed with leg traction is a technically feasible and safe procedure that enhances visualization of both femoral and acetabular cartilage surfaces. This technique helps separate joint surfaces and improves the detection of subtle cartilage lesions [32-34].
Most hip fractures can be easily diagnosed using radiographs [35]. In cases in which the diagnosis is unclear, MRI plays a crucial role in identifying fractures that may not be visible on radiographs. Its superior soft tissue contrast and ability to detect subtle bone marrow changes make MRI an invaluable tool for detecting occult fractures, particularly in areas with complex anatomies or when radiographic findings are inconclusive. This ability enhances early detection and guides appropriate treatment strategies, reducing the risk of complications [36].
Stress fractures, which result from the cumulative impact of repetitive microtrauma, are often difficult to detect on radiographs due to their non-displaced nature. These fractures usually present as subtle changes in the bone, making them challenging to identify on standard imaging. However, if repetitive stresses continue to affect the bone, the fracture may progress and eventually become displaced, at this point, it becomes more evident on radiographic images. The early detection of stress fractures is critical for preventing further damage and ensuring appropriate management [35, 36]. Stress fractures are categorized into two types: Fatigue and insufficiency. Fatigue fractures occur in healthy bones subjected to excessive or repetitive loading, accumulating microtrauma over time. In contrast, insufficiency fractures arise in bones weakened by underlying conditions, such as osteoporosis, making them susceptible to fracture even under normal loading conditions. Both types of fractures can be difficult to detect in the early stages but require careful management to prevent further injury and complications [36]. MRI is considered to be the most sensitive and accurate imaging modality for detecting and grading stress injuries. Its high resolution allows for the visualization of early bone marrow changes, soft tissue involvement, and subtle fractures that may not be apparent on radiographs. MRI’s ability to provide detailed images of bone and surrounding tissues makes it invaluable for assessing the severity of stress injuries and monitoring their progression, thus guiding appropriate treatment and preventing further complications [35].
Femoral neck stress fractures are classified into two types based on their location relative to the forces acting on the bone: Compression and tension-side fractures [37]. Compression-sided injuries, found at the inferomedial aspect of the femoral neck, are characterized by a low risk of displacement and are typically managed through conservative treatment. In contrast, tension-sided injuries located at the superolateral femoral neck are associated with a higher risk of displacement and often necessitate surgical intervention to prevent complications. Additionally, MRI is utilized to detect radiographically occult extensions of intertrochanteric or cervical fractures, particularly in cases involving known greater trochanteric fractures. This advanced imaging technique allows for more accurate assessment and treatment planning in complex fracture scenarios [38].
Multiple surgical and MRI-based classifications of labral tears exist; however, these classification systems exhibit poor agreement with one another. Discrepancies between the two approaches can complicate diagnosis and treatment planning, as each system may highlight different aspects of tear characteristics or location. This lack of consensus underscores the need for a more standardized and reliable classification system to enhance clinical decision-making and improve patient outcomes [39, 40].
On MRI, labral tears and detachments typically appear as linear hyperintense signals in fluid-sensitive sequences. MRA is considered the gold standard for evaluating the labrum, offering superior sensitivity and accuracy for detecting labral tears and detachments. The enhanced contrast provided by MRA allows for a clearer delineation of the labral structure, making it the most reliable imaging modality for assessing these injuries [36, 41]. On MRA, intrasubstance tears are characterized by contrast material within the labral substance, indicating disruption of labral tissue. In contrast, labral detachment is identified by the presence of contrast at the acetabular-labral interface, which signifies the separation of the labrum from the acetabulum. This distinct finding is a key diagnostic feature of labral detachment on MRA and provides valuable information for treatment planning [36].
Recent advances in medical imaging technologies
Three-dimensional (3D) imaging
In 2018, Professor Merloz summarized three decades of experience in image-guided orthopedic surgery, stating: “This recent, fascinating, and international journey, led by French teams at the forefront, is the result of collaboration across multiple scientific disciplines, including mathematics, computer science, radiology, physics, and medicine. Pre- or intraoperative imaging equips the surgeon with tools to visualize and interpret crucial information, facilitating the performance of the planned procedure with appropriate instruments, such as navigation, robotics, or personalized templates. The future of surgical navigation is influenced by the growing utilization of 3D intraoperative imaging and augmented reality technologies. This shift is already taking place, as evidenced by the publication of 2 688 articles in the past decade, which accounts for half of all publications on the subject [42].
The use of multislice CT (MSCT) in the operating room is limited by several factors. The equipment itself is large and not easily accommodated in surgical suites, and it is also a significant source of radiation. This restricts the types of procedures that can be performed in the CT room to simpler ones, such as biopsies or percutaneous procedures with linear trajectories, which are less invasive and impose fewer anesthetic challenges. More complex surgeries, such as those requiring fracture reduction or open implant insertion, are not feasible with MSCT in the operating room. However, MSCT provides very high-resolution images, which are crucial for procedures in sensitive body areas, such as neurosurgery. To address these limitations, newer MSCT units, such as AIRO® (Brainlab) and BodyTom (Samsung), have been developed specifically for operating rooms, making them more suitable for surgical settings. Cone-beam CT (CBCT) systems are advanced fluoroscopy units equipped with scintillation counters that enable the reconstruction of 3D images in the DICOM and Communications in Medicine format. Initially developed for use in dentistry, CBCT technology has since been adapted for various medical applications due to its ability to produce high-resolution images with lower radiation doses than traditional CT scanners. The compact nature of CBCT systems makes them particularly suitable for use in operating rooms, offering real-time imaging and facilitating more precise procedures [43, 44]. MSCT can be optimized for low-dose imaging, making it suitable for repeated use in CT-guided biopsies. However, CBCT has some limitations. The examination field is smaller due to the size of the detector, which provides less information about the surrounding soft tissues [45]. Additionally, this technology is more susceptible to noise from ray dispersion, reducing the contrast resolution. Truncation artifacts may also occur, and Hounsfield units cannot be used to characterize pixel density as in traditional CT. Despite these drawbacks, CBCT is particularly effective in orthopedic surgery, offering highly detailed images of bone structures. Early analog CBCT systems had the additional limitation of image distortion caused by the conical source projection in a 2D plane, requiring a calibration target and limiting the available working space. Modern flat-panel detector (FPD) technology has largely addressed this issue by digitizing images at the source, eliminating the need for a target. Modern FPD systems also avoid magnetic field distortion and provide a more extensive operating field, improving radiographic coverage and image resolution. For example, the Philips Veradius Unity mobile C-arm uses a 27×27-cm2 detector with 184-µm resolution, offering enhanced imaging capabilities while reducing radiation exposure to staff and patients. Nevertheless, CBCT, similar to MSCT, is susceptible to motion artifacts during image acquisition. Both technologies require patients to hold their breath, which can be challenging during procedures under local anesthesia. The new Surgivisio® system (eCential) addresses this issue by incorporating an acquisition coordinate system that allows imaging to be conducted during normal breathing, further improving the practicality of CBCT for surgical procedures [45].
Dynamic imaging
Hip arthrography is a pivotal diagnostic tool in managing Legg-Calvé-Perthes disease, offering critical insights that aid in tailoring treatment strategies to each patient’s specific needs. By providing real-time imaging of the hip joint, arthrography enables precise assessment of the femoral head’s congruence, extent of joint containment, and condition of the articular cartilage. This detailed visualization is particularly valuable for evaluating the dynamic relationship between the femoral head and acetabulum, which is essential for determining the optimal course of treatment. Moreover, hip arthrography assists in identifying subtle joint abnormalities that may not be apparent in conventional imaging modalities, such as plain radiographs or MRI. This technique is especially useful during surgical interventions, where it helps guide procedures, such as femoral or pelvic osteotomies, to ensure proper joint alignment and containment. As such, hip arthrography is an indispensable tool in the personalized management of Legg-Calvé-Perthes disease, contributing to improved clinical outcomes and preserving hip function over the long term [46-48]. In children with Legg-Calvé-Perthes disease, hip arthrography is a valuable diagnostic tool that provides dynamic imaging to evaluate critical aspects of joint morphology and function. This technique allows for detailed assessment of the congruency and containment of the femoral head within the acetabulum, which is pivotal in determining the stability and alignment of the hip joint. Additionally, hip arthrography is instrumental in excluding the presence of “hinge” abduction, a condition in which the lateral portion of the femoral head impinges on the acetabular rim during abduction, leading to restricted motion and joint incongruence. Identifying this abnormality is crucial because it often influences the choice of treatment, particularly when surgical interventions, such as femoral or pelvic osteotomies are being considered. Hip arthrography enhances the precision of treatment planning by offering real-time insights into hip biomechanics, ensuring that therapeutic strategies are customized to optimize joint function and long-term outcomes in patients with Legg-Calvé-Perthes disease [49]. Hinge abduction is a condition in which the femoral head cannot shift medially during abduction. This restriction occurs due to pronounced flattening of the femoral head, causing it to become obstructed by the lateral edge of the acetabulum, thereby limiting proper joint movement [48]. The primary goal of the management of Legg-Calvé-Perthes disease is to improve joint containment and congruity. Hip arthrography is crucial in achieving this by providing detailed information that helps identify patients who may benefit from surgical interventions such as periacetabular and intertrochanteric osteotomies. The insights gained from arthrography enable clinicians to make informed decisions about the suitability and timing of these procedures, optimize outcomes, and preserve hip function [49-51]. Identifying hinge abduction is crucial because addressing this condition is essential for ensuring proper development of the femoral head. Achieving this requires preserving adequate contact between the femoral head and the anatomically normal acetabulum. In numerous cases, this goal can be successfully attained through well-planned surgical interventions, which help restore joint congruence and promote optimal hip development [48]. Arthrography also facilitates the assessment of joint congruity during treatment strategies involving immobilization to promote epiphyseal healing. This imaging technique provides valuable insights into the alignment and stability of the joint, ensuring that immobilization effectively supports the healing process and maintains proper anatomical relationships [51].
MRI is a vital diagnostic modality for detecting early-stage or radiographically occult Legg-Calvé-Perthes disease because it provides detailed visualization of the extent of epiphyseal involvement. Its ability to capture soft tissue and bone marrow changes with high resolution makes it indispensable for early identification and comprehensive evaluation of the disease [52]. Dynamic hip examination using MRI is constrained by the enclosed design of conventional MRI systems, which limits its ability to evaluate joint function in various positions. However, the increasing availability of open-configuration MRI systems has addressed this limitation, enabling dynamic imaging of joints in multiple positions. This advancement allows for a more comprehensive assessment of joint mechanics and alignment, enhancing diagnostic and treatment planning capabilities.
Low-dose imaging systems
The incidence of hip preservation surgery among adolescents and young adults has steadily increased in recent years. Achieving favorable clinical outcomes and reducing the likelihood of revision surgeries depends heavily on comprehensive correction of the underlying osseous pathomorphology. Precise and thorough surgical intervention aimed at addressing bony deformities is essential for restoring joint functionality, alleviating pain, and preventing long-term complications, such as early onset osteoarthritis or joint instability. This underscores the importance of meticulous preoperative planning and advanced surgical techniques tailored to each patient’s unique anatomical and biomechanical challenges [53-57]. Plain radiographs have notable limitations in accurately capturing the 3D characteristics of hip morphology. These limitations become particularly evident when attempting to precisely localize and evaluate cam deformities or acetabular morphology, especially in the context of variable pelvic tilt and rotation. While some surgeons continue to rely exclusively on plain radiographs in combination with intraoperative dynamic fluoroscopic assessments to evaluate bony morphology, there is a growing shift toward incorporating CT scans into the preoperative assessment process. CT imaging provides superior visualization and quantitative data, offering advanced 3D reconstructions that enhance our understanding of the complex anatomy of the pelvis, acetabulum, and femur. This level of detail is invaluable for identifying subtle deformities and obtaining a more accurate assessment of the underlying pathomorphology. Additionally, CT scans help resolve potential diagnostic ambiguities associated with plain radiographs, such as false-positive findings, including the crossover sign or posterior wall sign. They also enable clinicians to account for and adjust pelvic tilt or rotation changes, which can significantly affect the accuracy of conventional radiographic interpretation. As such, integrating CT imaging into preoperative planning is becoming an increasingly valuable tool for achieving precise diagnoses and optimizing surgical outcomes in hip preservation surgery [58]. Despite the enhanced information provided by CT scans, one of the primary drawbacks is increased radiation exposure, which poses particular concern for hip-preservation surgeons treating adolescents and young adults who are more sensitive to radiation. However, recent technological advancements and the development of refined scanning protocols have led to substantial reductions in the radiation doses during these scans. Low-dose CT imaging protocols have been successfully implemented across pediatric and adult populations, facilitating various diagnostic and surgical procedures with minimal risks. Nevertheless, the application of low-dose CT specifically for preoperative planning in hip preservation surgeries remains insufficiently established. Although the potential benefits of reducing radiation exposure are clear, there is a need for further research and clinical validation to determine the efficacy and safety of low-dose hip CT scans in surgical planning. This includes exploring whether the reduced radiation dose still provides the necessary detail and accuracy for optimal preoperative assessment, especially in the delicate patient population requiring hip preservation procedures. Therefore, further studies are essential to evaluate the feasibility and reliability of low-dose CT as a standard tool for hip preservation surgical planning [59-64].
Expected effects of artificial intelligence (AI) in hip fracture diagnosis
The swift identification of non-displaced hip fractures can be challenging for clinicians and may necessitate additional imaging techniques, such as radiography, bone scans, CT, or MRI. However, these supplementary tests may not be readily accessible at all healthcare facilities. Moreover, factors, such as bone demineralization and overlying soft tissues can complicate the accurate diagnosis of hip fractures [65]. Delayed diagnosis and treatment of hip fractures can result in complications, such as mal:union:, osteonecrosis, and arthritis [66]. Additionally, with the increasing number of imaging and radiological examinations, radiology departments often cannot report all acquired radiographs promptly [67]. As a result, several studies have been conducted on using machine learning to detect hip fractures [65, 66, 68-72]. Early diagnosis of hip fractures using AI algorithms in clinical practice could help reduce medical costs, enable more effective preventive measures, and improve the overall quality of healthcare [71]. AI-driven early diagnosis of hip fractures also enhances resource allocation, reduces unnecessary consultations, and accelerates patient disposition. This allows physicians to focus on more complex tasks in high-volume clinical settings. However, there is a lack of sufficient reports on the effectiveness of AI algorithms for early diagnosis of hip fractures, suggesting that further research is necessary.
AI versus human
The results of the studies included in the analysis showed that the accuracy of hip fracture diagnosis using AI algorithms was over 90%, with the exception of Beyaz et al. Additionally, the area under the curve (AUC) for fracture diagnosis consistently exceeded 0.9, indicating a very high diagnostic performance [70]. In a comparative study between AI and human diagnosis of hip fractures, the diagnostic accuracy of AI was found to be higher. For instance, Urakawa et al. developed an AI model that detected intertrochanteric fractures with an accuracy of 95.5% and AUC of 0.984, demonstrating the high performance of AI in identifying these fractures [73]. The AI model developed by Urakawa et al. outperformed the human diagnostic accuracy of 92.2%, with an AUC of 0.969. Adams et al. reported a conventional neural network model for diagnosing femoral neck fractures, achieving an accuracy range of 88.1% to 94.4%. This highlights the potential of AI to surpass human performance in certain diagnostic contexts [74]. These values are also comparable to the diagnostic accuracies of experts and residents, which were 93.5% and 92.9%, respectively. However, in studies conducted by Cheng et al. [71] and Sato et al. [75], the human diagnostic accuracy was lower than that of the AI algorithm. This further underscores the potential of AI to achieve higher diagnostic performance in detecting hip fractures [71]. Nevertheless, whether AI can fully replace humans in hip fracture diagnosis remains uncertain. Bae et al. utilized AI to diagnose femoral neck fractures after training the algorithm with 4189 images, achieving a diagnostic accuracy of 97.1%. However, they noted that detecting non-displaced fractures of the femoral neck remains challenging despite the high overall diagnostic accuracy of the AI model. This highlights a limitation of current AI systems in addressing specific fracture types, suggesting that while AI can be highly accurate, human expertise may still be necessary for certain cases [72]. This suggests that AI may have limitations in cases where it has not been adequately trained or lacks sufficient learning. Additionally, since the AI systems examined in the study are not integrated with other clinical information, they cannot yet replicate the clinical judgment of a human physician, such as the ability to suspect occult fractures based on a patient’s overall condition. Mawatari et al. also argued that since the AUC values for AI-assisted expert diagnoses were higher than those for the AI algorithm alone, a valid diagnosis could not be obtained from radiographs alone. This highlights that the effectiveness of AI diagnosis is still heavily influenced by the quality and scope of the AI algorithm [65]. Therefore, AI algorithms cannot entirely replace human intelligence in the clinical environment. However, they can complement and enhance physicians’ capabilities and knowledge. A potential concern is the increasing reliance on AI for hip fracture detection, as it may reduce the need for doctors to make their clinical judgments. This reliance can stem from the difficulty and time constraints clinicians face in synthesizing the results of various examinations performed in direct patient interactions. Balancing AI assistance with clinical expertise remains essential for comprehensive patient care [71]. To address this issue, Cheng et al. developed a system in which the areas identified by AI for hip fracture detection were highlighted and displayed, allowing physicians to review the results of the AI algorithm and make the final clinical judgment. This approach enables clinicians to integrate AI findings with their expertise, ensuring that the decision-making process remains collaborative and informed by the algorithm and physician’s assessment [71]. With advancements in technology, AI algorithms are expected to continue evolving, leading to increased reliance on AI. However, this growing dependence on AI may present challenges in balancing between human judgment and algorithmic assistance. Further research is essential to explore solutions to these challenges, ensuring that AI can complement, rather than replace, human expertise in clinical decision-making.
AI deep learning for hip fracture
Because deep learning algorithms in AI automatically and adaptively learn features from data, the quality and quantity of datasets used for training are critical. Large, clean, and well-labeled datasets ensure that AI models can effectively learn and generalize from diverse clinical scenarios, ultimately leading to more accurate and reliable performance in real-world applications [70]. The accuracy of AI in detecting hip fractures is significantly influenced by the number of images used for training the model. A larger dataset allows the AI system to learn from various cases, improving its ability to generalize and detect fractures across different patient populations and imaging conditions. As the number of images increases, the AI’s performance typically improves because it becomes better equipped to identify subtle patterns and variations in the data indicative of hip fractures [75]. Mutasa et al. created 9063 augmented images by combining 737 hip fracture images with 326 normal images from a dataset of 550 patients. Beyaz et al. also generated 2 106 augmented images from 234 radiographs of 65 patients. By augmenting the data, these studies aimed to enhance the AI models’ ability to learn from a more diverse set of images, thereby improving the accuracy and robustness of fracture detection algorithms. This augmentation process helps the model to generalize better across different patient demographics and imaging conditions [69, 70]. Yu et al. reported that detecting a distinct fracture line or cortical angular deformity in femoral neck fractures is relatively straightforward using a single radiographic view. However, a larger sample size is necessary for accurate detection due to the wide spectrum of fracture morphologies for intertrochanteric fractures, which often involve complex and multiple fracture lines. This suggests that although some fracture types are easier to identify with a single radiograph, more intricate fractures require a broader dataset to enhance diagnostic accuracy, particularly when using AI algorithms for detection [68]. Additionally, factors, such as soft-tissue shading and variations in femur alignment, can impact the ability of AI to detect fractures accurately. Soft tissue shadows can obscure fracture lines, making it more difficult for AI to distinguish between fractures and surrounding structures. Similarly, variations in femoral alignment, such as differences in positioning or angle during imaging, can alter the appearance of fractures and potentially lead to false positives or negatives in AI detection. These challenges highlight the need for careful image acquisition and consideration of such factors when developing and using AI models for hip fracture diagnosis [76]. Additionally, factors, such as soft-tissue shading and variations in femur alignment, can impact the ability of AI to detect fractures accurately. Soft tissue shadows can obscure fracture lines, making it more difficult for AI to distinguish between fractures and surrounding structures. Similarly, variations in femur alignment, such as differences in positioning or angle during imaging, can alter the appearance of fractures and potentially lead to false positives or negatives in AI detection. These challenges highlight the need for careful image acquisition and consideration of such factors when developing and using AI models for hip fracture diagnosis [66]. In contrast, Yoon et al. reported that the use of both CT images and radiographs for the classification of intertrochanteric fractures significantly reduced the time required for fracture classification. This approach also assisted in planning more accurate surgical procedures. By providing more detailed and comprehensive imaging data, CT scans helped clarify fracture patterns, enabling surgeons to make better-informed decisions regarding the appropriate treatment strategy. This integration of multiple imaging modalities highlights the value of combining radiographs with advanced techniques, such as CT, to improve the efficiency and accuracy of hip fracture management [77]. Mawatari et al. used MRI and CT for hip fracture detection [65]. However, this approach has the disadvantage of incurring additional costs and the challenge of obtaining a normal lateral view of the hip in some cases. Despite these limitations, AI offers significant potential for diagnosing and classifying diseases because it rapidly processes large volumes of patient data. By efficiently analyzing complex imaging data, AI can enhance diagnostic accuracy, reduce the time spent on classification tasks, and support clinical decision-making, while minimizing the dependency on costly and difficult-to-obtain imaging views [78]. The usefulness of AI is being actively studied in trauma prediction, a field characterized by significant individual variability in the number and severity of injuries. This variability arises from the complex interaction of various external and internal factors, such as the mechanism of injury, patient demographics, and pre-existing health conditions. By processing vast amounts of data, AI algorithms can identify patterns and predict outcomes more accurately than traditional methods, helping anticipate the trauma’s extent and guide clinical interventions. This potential for personalized trauma care can significantly improve patient outcomes and optimize resource allocation in trauma settings [79].
Innovative molecular imaging techniques
Positron emission tomography (PET)/CT and PET/MRI
Since the 1960s, radionuclide bone scanning h::as char::acterized a wide range of bone pathologies. In addition to their ability to detect bone metastases from various tumors well before other radiographic modalities of that era, bone scans gained recognition for their superior sensitivity in identifying occult fractures, stress fractures, osteoid osteomas, and other musculoskeletal conditions of interest to orthopedists. While some nuclear medicine practices developed significant expertise in diagnosing soft tissue injuries, most studies have focused primarily on evaluating bony pathology. Therefore, bone scans has become an essential diagnostic tool in orthopedics, particularly for conditions that are difficult to detect using conventional radiography [80]. Several factors have contributed to the gradual reduction in the use of bone scanning in orthopedics. Many injuries requiring surgical intervention primarily involve soft tissues around the joints. MRI has advanced significantly over the past three decades, providing clear delineation of most soft tissue pathologies. While MRI’s specificity for bone conditions is somewhat limited, it can surpass bone scanning in certain cases, particularly in older patients with acute hip trauma, because it often detects fractures days before a bone scan shows positive results [81]. MRI compensates for its limited specificity for bone pathology by providing exceptional anatomical details. In many nuclear medicine practices, bone scanning in orthopedics has been primarily reserved for patients with stress injuries or certain conditions in which other imaging modalities have failed to diagnose definitively. The sensitivity of bone scanning can be enhanced by incorporating single-photon emission computed tomography; however, the absence of detailed anatomical information often makes it challenging to interpret results confidently [82].
PET has historically been underused in musculoskeletal imaging due to several factors, including the high effectiveness of musculoskeletal MRI, the limited spatial resolution of PET, and the lack of reimbursement for such studies. However, with advancements in PET/CT and PET/MRI technology over the past decade, along with a deeper understanding of the pathophysiology of musculoskeletal diseases, PET is emerging as a promising primary or complementary imaging modality for managing rheumatologic and orthopedic conditions. The low metabolic activity of bones and tendons can be beneficial and challenging for PET/CT evaluation. However, these structures’ minimal physiological background activity enhances the target-to-background ratio when imaging disease processes, which is a significant advantage. In contrast, non-neoplastic, non-inflammatory musculoskeletal conditions, such as traumatic injuries, tendon, and ligament abnormalities, can exhibit low metabolic activity, making them less detectable by PET [83, 84].
PET/CT versus PET/MRI
PET/MRI, as a hybrid imaging modality, offers several advantages over PET/CT, including reduced radiation exposure for patients and superior soft-tissue contrast. This higher soft-tissue resolution is particularly beneficial for the diagnostic assessment of tendinous and ligamentous pathological conditions, where detailed visualization of soft tissues is essential [85]. Most importantly, PET/MRI outperforms PET/CT in terms of superior co-registration of PET data with MRI, and enhanced motion correction based on MRI. This combination leads to more accurate and reliable imaging, particularly in assessing dynamic musculoskeletal structures where motion artifacts can be problematic [85]. PET/MRI has been regarded as a comprehensive solution for the diagnostic management of patients with musculoskeletal disorders, as it allows for the simultaneous acquisition of multisequence, multiparametric MRI, and whole-body PET imaging. This makes it particularly efficient for patients requiring extensive MRI workups. However, PET/CT still offers superior attenuation correction, is more widely accessible, and is less expensive. A key limitation of PET/MRI is that the standardized uptake values derived from PET/MRI can be scanner-dependent, complicating direct comparisons between PET/CT and PET/MRI data [83, 85].
Studies have demonstrated that in the assessment of suspected knee and hip prosthetic infections, FDG PET offers superior accuracy compared to the combination of bone marrow and leukocyte scintigraphy, with accuracy rates ranging from 80% to 98%, versus 25% to 96% for the latter approach [86]. The sensitivity of combined bone marrow and leukocyte scintigraphy is 30%–35%. However, studies have shown that FDG PET, when used to evaluate patients with suspected hip prosthetic infections, demonstrates similar sensitivity but slightly lower specificity than combined bone marrow and leukocyte scintigraphy [87]. It has also been shown that PET provides no additional benefit over combined bone marrow and leukocyte scintigraphy when evaluating total knee arthroplasties [88].
Despite conflicting data regarding the diagnostic performance of FDG PET in periprosthetic infections compared to imaging with single-photon emitters, PET presents several advantages. These include time savings (as it does not require dual-image acquisition, such as dual-tracer techniques), higher spatial resolution, and a better cost and safety profile compared to the complexity and risks associated with using labeled white blood cells, which involve handling blood products. As a result, FDG PET/CT is a suitable diagnostic imaging method for patients with suspected hardware infections [89].
PET/CT has been studied for the management of patients with suspected implant-related infections of the tibia after osteosynthesis. FDG PET/CT assisted in clinical decision-making by distinguishing between infected non:union:, aseptic non:union:, soft-tissue infection, and chronic osteomyelitis. The overall sensitivity of FDG PET/CT for identifying osseous infections was 85.7%, with a specificity of 100%. This highlights the potential of FDG PET/CT to accurately diagnose implant-related infections and guide treatment decisions [89].
The role of nanotechnology in early detection
Nanotechnology is the science of designing, manipulating, and engineering materials at molecular and atomic scales. In bone regeneration, this technology is used to create new bone tissue or enhance the healing of existing bone tissue by utilizing materials that stimulate and support bone growth. Owing to their small size and unique properties, nanomaterials can be integrated into scaffolds, coatings, or drug delivery systems to improve bone regeneration, promote cell growth, and accelerate the healing process in fractures or bone defects [90, 91]. Nanotechnology is increasingly employed in bone regeneration to develop more precise, effective, and targeted materials that enhance bone growth. By manipulating materials at the nanoscale, scientists can create scaffolds, coatings, and drug delivery systems that are better suited to mimic the natural bone environment. These nanomaterials improve cell adhesion, proliferation, and differentiation, thereby accelerating bone healing and regeneration. Additionally, their small size allows for enhanced bioactivity, better integration with surrounding tissues, and more controlled delivery of therapeutic agents, ultimately leading to more successful bone repair and regeneration [92]. For example, researchers are investigating the use of nanoparticles to deliver drugs or other therapeutic molecules directly to areas in need of bone regeneration, enhancing the treatment’s effectiveness. These nanoparticles can be engineered to target specific sites, such as bone fractures or areas of degeneration, allowing for more precise and localized treatment. By controlling the release of growth factors, osteoinductive molecules, or other regenerative agents, nanoparticles ensure that these compounds are delivered at optimal concentrations at the right time, maximizing their therapeutic potential and minimizing side effects. This targeted approach holds great promise for improving the outcomes of bone healing and regeneration therapies [92]. Nanoparticles can also be utilized to create scaffolds that replicate the natural structure of bones, providing a framework to guide and support new bone growth. These nanostructured scaffolds can enhance cell adhesion, proliferation, and differentiation and promote more efficient bone regeneration. Moreover, advancements in 3D printing technology, incorporating nanoscale materials, enable the fabrication of highly precise, customized implants tailored to individual patients’ anatomical needs. These implants can be designed to match the patient’s bone structure, optimize the healing process, and ensure better integration with the surrounding tissue. Together, nanotechnology and 3D printing innovations hold great potential for revolutionizing bone regeneration therapies, offering more effective and personalized treatments for bone-related injuries and conditions [90]. Bone weakening and dysfunction are significant health concerns, particularly in osteoporosis, fractures, and age-related bone degeneration. These challenges have prompted nanotechnologists to focus on the potential of nanotechnology in addressing bone-related issues, marking it a crucial area of research in the intersection of nanotechnology and medicine. Studies are increasingly exploring how nanotechnology can aid bone formation, structuring, and repair. Nanotechnology offers the potential to develop advanced biomaterials that can mimic the natural bone structure at the nanoscale, enhance bone strength, and promote regeneration. Researchers are investigating the use of nanoparticles to deliver therapeutic agents directly to bone tissue, stimulate bone formation, and improve bone density. Nanostructured scaffolds are being developed to provide an ideal environment for bone cell growth, ultimately supporting the formation of new bone tissue and enhancing the healing process after fractures or surgeries. These advancements can revolutionize treatments for conditions that weaken bones and promote more effective strategies for bone regeneration and repair [90]. Scientists are working on developing bone graft substitutes using nanostructured materials that closely mimic the natural properties of bones. These materials are biocompatible, allowing them to be seamlessly integrated into the body without triggering an immune response. If these studies succeed, they have the potential to revolutionize regenerative medicine by offering more effective solutions for repairing damaged bones and tissues. Creating bone grafts that promote natural bone regeneration can significantly improve the healing process of fractures, bone defects, and conditions, such as osteoporosis, offering a promising alternative to traditional bone repair methods. The development of nanostructured grafts is a step toward advanced regenerative technologies, where the material serves as a scaffold for bone growth and actively supports cellular activity, promoting healing and tissue regeneration. This could lead to improved outcomes in treating bone injuries, potentially reducing the need for long recovery times and offering patients more effective treatment options for broken or damaged bone and muscle fragments [93]. Principal investigations into biomineralization are focused on reducing the particle size of bone materials to enhance their interaction with collagen fibers. This approach aims to replicate the natural process by which bones mineralize and incorporate minerals into a collagen matrix to create a strong and functional composite structure. By reducing the particle size of bone materials, researchers can increase the surface area and improve the bonding efficiency with collagen fibers, allowing for better integration and stability within the bone matrix. This process also seeks to mimic the crystalline properties of bones, which are essential for their strength and resilience. Coupling fine mineral particles with collagen fibers can improve the material’s mechanical properties, making it more effective in supporting bone regeneration and repair. Additionally, this method can potentially enhance the material’s bioactivity, encouraging cellular activity that promotes bone healing and regeneration. Ultimately, these investigations aim to develop advanced bone substitutes that more closely resemble natural bone tissue in terms of structure and function. This can lead to better outcomes in regenerative medicine, offering more effective treatments for bone fractures, defects, and degenerative conditions [92]. The aim is to develop a composition that effectively penetrates damaged bone regions and possesses tailored mechanical properties to transform the field of osteology and advance bone tissue engineering [92]. Similar research is underway to develop artificial joints with nanoscale collagen-mimicking coatings for the knee and hip, which help stabilize the bone-formation process by osteoblasts [94, 95]. In summary, the application of nanotechnology in bone regeneration offers significant potential to enhance bone repair and regeneration outcomes, leading to faster healing, stronger bones, and fewer complications.
Conclusion
In conclusion, recent advancements in imaging technologies have offered significant improvements in the diagnosis of hip disorders, enabling more accurate, timely, and efficient assessments. Conventional imaging techniques remain vital, but emerging technologies, such as AI, 3D and dynamic imaging, and molecular imaging, are expanding diagnostic capabilities. AI has shown promising potential for the rapid and accurate diagnosis of hip fractures, particularly non-displaced fractures, which can be difficult for clinicians to detect. Additionally, the application of PET/CT and PET/MRI to assess metabolic changes and the role of nanotechnology in early detection represent groundbreaking steps. However, challenges related to cost, accessibility, and integration into clinical workflows must be addressed for these technologies to reach their full potential. Continued research and innovation are necessary to optimize these tools for routine clinical use, ultimately improving patient outcomes in managing hip disorders.
Limitations
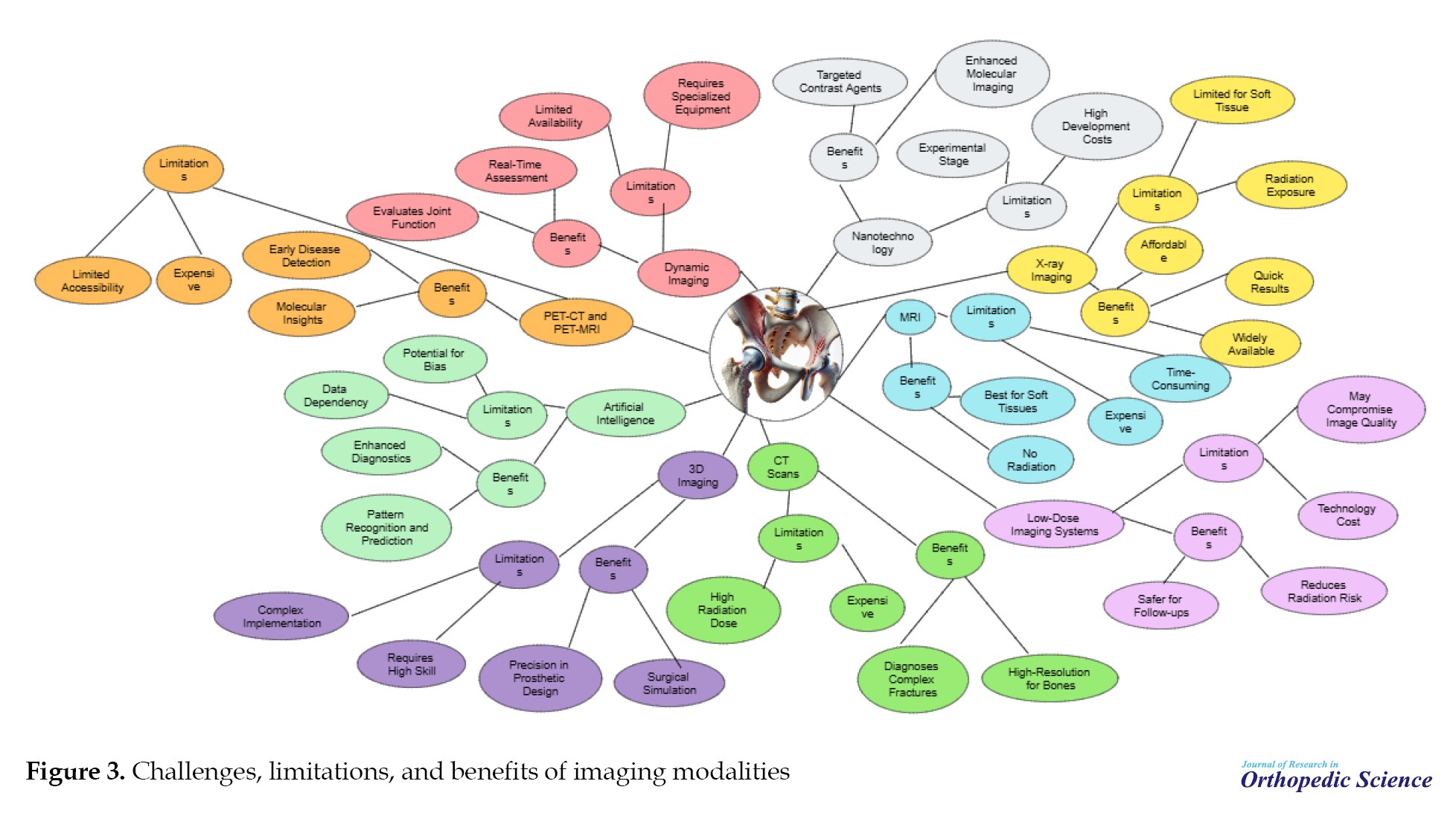
In addition to the advancements above, each imaging modality has its own set of challenges, advantages, and limitations. These factors are outlined and discussed in detail (Figure 3).
Ethical Considerations
Compliance with ethical guidelines
There were no ethical considerations to be considered in this research.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Conceptualization: Karim Pisoudeh, and Khatere Mokhtari; Methodology: Siamak Kazemi;Data collection: Karim Pisoudeh, Khatere Mokhtari, and Siamak Kazemi; Data analysis: Khatere Mokhtari and Siamak Kazemi; Investigation, and writing: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to express their gratitude to the Department of Orthopedics, Bone and Joint Reconstruction Research Center, School of Medicine, Shafayahyaeian Hospital, Iran University of Medical Sciences, Tehran, Iran, for their invaluable support in conducting this research. Additionally, we would like to thank the Department of Cellular and Molecular Biology and Microbiology, Faculty of Biological Science and Technology, University of Isfahan, Isfahan, Iran, for their assistance in providing the necessary expertise.
References
Hip disorders in medicine
Hip pain affects approximately 10% of the general population, and its prevalence progressively increases with age [1]. A published study revealed that 14.3% of adults experienced significant hip pain on most days within the preceding six weeks [2]. Hip pain is often associated with difficulty performing basic movements, such as sitting and standing, which can lead to chronic pain and adversely affect functional capacity and overall quality of life. Diagnosing hip pain can be complex due to potential referred pain from sources, such as the spine or knee, as well as conditions, such as trauma, tumors, abdominal or hernial issues, joint arthropathies, muscular disorders, and neuropathies [3].
The hip joint, a ball-and-socket synovial joint, serves as a critical structure for weight transfer between the upper and lower body while facilitating movement across multiple planes. Although inherently shallow, the joint gains additional depth and stability from the labrum, a ring of fibrocartilage that encircles the acetabular rim [3]. The hyaline cartilage envelops the articular surfaces of the hip joint and efficiently absorbs and dissipates shear and compressive forces during movement. The joint’s structural integrity is reinforced by ligaments, with the iliofemoral and pubofemoral ligaments providing anterior support and the ischiofemoral ligament stabilizing posteriorly [4]. The hip joint is encircled by numerous muscle groups that facilitate a broad range of movements and contribute to versatility and functional mobility [4]. The trochanteric, iliopsoas, gluteus medius, and ischiogluteal bursae serve as cushions between the bones and surrounding tendons of the hip joint, reducing friction and facilitating smooth movement. The hip joint receives its nerve supply from the articular branches of the quadratus femoris nerve, obturator nerve, femoral nerve, sciatic nerve, and nerves supplying nearby muscles, including the superior and inferior gluteal nerves [5]. Multiple nerves’ complex innervation of the hip joint makes it challenging to differentiate primary hip pain from radicular pain originating from the lumbar spine [3] (Figure 1).
The role of Imaging technologies in improving diagnosis
Imaging technologies play a pivotal role in diagnosing, assessing, and managing hip disorders by providing detailed insights into the anatomical, functional, and metabolic aspects of the hip joint. These technologies significantly enhance clinicians’ ability to detect abnormalities, monitor disease progression, and plan effective treatments [6, 7, 8]. Key ways imaging technologies contribute to improved diagnosis (Figure 2). This mini-review aims to provide an overview of recent advancements in imaging technologies and their applications in diagnosing hip disorders. Since hip pathologies significantly impact patient mobility and quality of life, timely and accurate diagnosis is crucial for effective management. This article examines how innovations in imaging techniques, from traditional modalities to cutting-edge technologies, have enhanced the detection, evaluation, and treatment planning for hip disorders.
Conventional imaging technologies for diagnosing hip disorders
X-ray imaging
Despite significant advancements in three-dimensional (3D) medical imaging technologies, planar x-ray imaging continues to be widely utilized in routine clinical practice. Traditional x-ray devices are relatively cost-effective and facilitate convenient image acquisition. However, accurate interpretation of x-ray radiographs remains challenging. This is primarily due to the projection of 3D anatomical structures onto a 2D plane, resulting in a loss of depth perception. Additionally, the radiographic magnification factor is typically approximated. Although newer generations of x-ray devices can standardize image acquisition, accurately estimating the magnification remains a significant challenge. X-ray radiographs are widely used in clinical settings for various purposes, including diagnostic assessment, pre-operative planning, and post-operative evaluation. Despite advancements in imaging technology, magnification distortion still requires careful consideration when interpreting radiographs for precise measurements and treatment decisions. Various methods have been proposed to enhance the accuracy [9-11]. A coin placed on the patient’s skin during x-ray acquisition was utilized as the reference object [9]. A prospective study demonstrated that the correct hip prosthesis size estimation can be improved from 59.4% to 68.8% when using a coin as a reference object, compared to using a caliper [10]. They showed that the use of a coin as a reference object allowed for a more accurate estimation of the radiographic magnification. They proposed a method utilizing a spherical object, which is adjustable in height and must be placed between the patient’s legs during imaging [11-14]. They employed a precise calibration method alongside the triangulation of manually identified points to reconstruct the spine and rib cage from biplanar x-ray images [13].
Computed tomography (CT) scans
In CT scanning, a series of x-ray beams and detectors are arranged in a circular configuration around the patient, who is positioned at the center of this circle. The x-ray beam passes through the patient with either the beam or detector rotating, generating complex images. These images are then mathematically reconstructed using a computer, enabling high-quality CT scan details. The data collected by the detectors are stored on tape and processed by a computer to create a cross-sectional image [15, 16]. This method differs significantly from the standard frontal and sagittal planes produced using traditional x-ray films, where the beam passes through the patient to a plate on the opposite side. Each structure along the beam’s path is captured in CT scanning, with denser structures masking those of lower densities. The cross-sectional images can be adjusted in thickness, with modern machines capable of producing slices as thin as 0.5 cm. These thin slices help eliminate image overlap, offering a clearer representation of the structures being defined. Once the image is captured, mathematical reconstructions allow for adjustments in image density using data stored on a computer tape. The operator can enhance structures of interest and exclude others, enabling selective examination of soft tissues, fluid collections, bone, or air densities without additional patient exposure. This capability is particularly valuable for assessing the musculoskeletal system, where bony structures are centrally located and surrounded by soft tissues. The complex three-dimensional anatomy of structures, such as the pelvis or spine, complicates the evaluation of bone and intra-articular injuries [17, 18]. Many musculoskeletal soft-tissue tumors, such as sarcomas, have tissue densities that closely resemble the surrounding tissues, making them undetectable on standard x-ray films. In these cases, alternative techniques, such as arteriography and radiography have been developed to help identify them, albeit with limited success. CT scanning addresses several of these limitations. However, CT scanning has some drawbacks. First, current models are less capable of distinguishing between two closely spaced points compared to routine x-ray films, although this issue is being gradually improved with further advancements. Second, the radiation dose from a CT scan is relatively high, ranging from one to two rads per slice or eight-ten rads per study. This is a significant dose that should be considered when ordering the procedure. The radiation dose increases when finer detail is needed but can be reduced if image quality is less critical (for example, in studies focusing on bone density). Finally, CT scans are expensive. Despite the high cost of machines (often approaching $750000), their extensive use in fields, such as medicine, surgery, neurology, and neurosurgery has proven to be cost-effective when applied appropriately. Due to their increasing utilization, the cost per study has significantly decreased over the years, and regional access has further helped reduce costs [19, 20]. Despite these limitations, CT scanners have become essential tools in specific orthopedic situations and should be considered in diagnosing musculoskeletal disorders [21, 22]. Radiographic evaluation of trauma to the femoral head and acetabulum traditionally involves anteroposterior and lateral x-rays, oblique views, and tomography. However, these methods can be hindered by the significant overlap of structures anterior and posterior to the hip, requiring correlation of data from various views to understand the fracture’s anatomy. As noted by Epstein, loose fragments within the joint can affect functional outcomes and should be removed if present. Cross-sectional imaging provided by CT scanning proved highly effective in visualizing the third dimension of hip trauma. One of the key advantages of CT scanning is its ability to detect subtle density changes, which helps identify intraarticular fragments, minor fractures, and dome fractures that may not be visible on routine x-rays or tomograms. The CT scan provides a clear view of the superior margin of the acetabulum, pelvic wall medial to the acetabulum and femoral head, and femoral head and neck because sections are obtained serially from cranial to caudal. This allows for precise assessment of the relationships between these structures, such as medial migration of the femoral head following a fracture of the central acetabulum. Since CT scans are performed with the patient in a supine position, traction can be maintained during the procedure, enabling the assessment of the effectiveness of traction in reducing the fracture. Moreover, the absence of oblique positioning makes CT scanning more comfortable for patients than oblique positioning. Additionally, complex fractures of the extraarticular portion of the pelvic bones, including sacroiliac joint disruptions, can be effectively evaluated using CT [21, 23].
Magnetic resonance imaging (MRI)
On MRI, the normal femoral head in adults typically demonstrates homogeneous high signal intensity on T1-weighted sequences, a characteristic attributed to its fatty marrow content. In contrast, the femoral neck and intertrochanteric region showed lower signal intensity on T1-weighted images due to red marrow and a reduced amount of fatty marrow. Articular hyaline cartilage, composed of a complex mixture of water, collagen, and proteoglycans, is crucial for distributing forces, absorbing pressure, and facilitating smooth gliding of the bony structures within the joint, thereby ensuring optimal joint function and mobility [24]. The entire femoral head is encased in articular hyaline cartilage, except a small area at the fovea that lacks cartilage. On MRI, normal articular hyaline cartilage typically presents with intermediate to high signal intensity on fluid-sensitive sequences, reflecting its water content. This characteristic indicates its crucial role in joint function, as cartilage facilitates smooth movement, reduces friction, and helps distribute mechanical loads across the joint surfaces [25, 26].
Although the normal acetabular labrum is typically hypointense and triangular, there is variability in both its morphological appearance and signal intensity among asymptomatic individuals. This variability can make interpretation more challenging because the labrum may present slight differences in shape, size, and signal characteristics in healthy individuals [27]. Intermediate-to-high signal intensity within the labral substance on MRI may result from small intralabral fibrovascular bundles. Additionally, increased signal intensity in the labrum on T1-weighted images can be attributed to the “magic angle” effect, which occurs when structures with a collagen-rich composition, such as the labrum, are imaged at a specific angle relative to the magnetic field, causing an increase in signal intensity [28]. A normal, mildly hyperintense 1-2 mm transition zone can be observed at the chondrolabral junction on MRI. This zone represents the interface between the articular hyaline cartilage and labrum, where there is typically a gradual change in signal intensity due to differences in tissue composition, such as the transition from cartilage to fibrocartilage [24]. Normal cartilage can “undercut” the labrum at the chondrolabral junction, which appears as a smooth focus of intermediate-to-high signal intensity on MRI. This area isointense to the cartilage and is situated between the labrum and acetabular rim. It represents a natural anatomical feature where the cartilage slightly extends beneath the labrum, contributing to joint stability and function [26]. A sublabral sulcus is a fluid-filled cleft located at the chondrolabral junction and is typically found anteroinferiorly at the four o’clock position. It is considered a normal variant in many cases. Additionally, a normal labro-ligamentous sulcus may be observed at the junction between the labrum and transverse acetabular ligament. As individuals age, the labrum may undergo degenerative changes, resulting in blunting, signal alterations, or complete absence of the labrum in some instances [29].
Magnetic resonance arthrography (MRA) is the current gold standard for assessing the acetabular labrum and hip hyaline articular cartilage. This technique involves the injection of contrast material into the joint, which helps separate the internal structures, allowing for clear visualization of the labrum and cartilage. The contrast extends into labral tears or chondral defects, making it highly effective for detecting these abnormalities and providing detailed images of the joint’s soft tissues [28, 30]. MRA offers higher diagnostic accuracy than non-contrast MRI for detecting acetabular cartilage lesions, whether performed at 1.5T or 3T. Using contrast material in MRA enhances the differentiation of cartilage defects and labral tears, providing more detailed and clearer images than standard non-contrast MRI, which improves the overall detection of joint abnormalities [31]. Despite the use of intra-articular contrast in MRA, the cartilages of the acetabulum and femoral head are often not delineated, making it challenging to visualize small lesions. However, hip MRA performed with leg traction is a technically feasible and safe procedure that enhances visualization of both femoral and acetabular cartilage surfaces. This technique helps separate joint surfaces and improves the detection of subtle cartilage lesions [32-34].
Most hip fractures can be easily diagnosed using radiographs [35]. In cases in which the diagnosis is unclear, MRI plays a crucial role in identifying fractures that may not be visible on radiographs. Its superior soft tissue contrast and ability to detect subtle bone marrow changes make MRI an invaluable tool for detecting occult fractures, particularly in areas with complex anatomies or when radiographic findings are inconclusive. This ability enhances early detection and guides appropriate treatment strategies, reducing the risk of complications [36].
Stress fractures, which result from the cumulative impact of repetitive microtrauma, are often difficult to detect on radiographs due to their non-displaced nature. These fractures usually present as subtle changes in the bone, making them challenging to identify on standard imaging. However, if repetitive stresses continue to affect the bone, the fracture may progress and eventually become displaced, at this point, it becomes more evident on radiographic images. The early detection of stress fractures is critical for preventing further damage and ensuring appropriate management [35, 36]. Stress fractures are categorized into two types: Fatigue and insufficiency. Fatigue fractures occur in healthy bones subjected to excessive or repetitive loading, accumulating microtrauma over time. In contrast, insufficiency fractures arise in bones weakened by underlying conditions, such as osteoporosis, making them susceptible to fracture even under normal loading conditions. Both types of fractures can be difficult to detect in the early stages but require careful management to prevent further injury and complications [36]. MRI is considered to be the most sensitive and accurate imaging modality for detecting and grading stress injuries. Its high resolution allows for the visualization of early bone marrow changes, soft tissue involvement, and subtle fractures that may not be apparent on radiographs. MRI’s ability to provide detailed images of bone and surrounding tissues makes it invaluable for assessing the severity of stress injuries and monitoring their progression, thus guiding appropriate treatment and preventing further complications [35].
Femoral neck stress fractures are classified into two types based on their location relative to the forces acting on the bone: Compression and tension-side fractures [37]. Compression-sided injuries, found at the inferomedial aspect of the femoral neck, are characterized by a low risk of displacement and are typically managed through conservative treatment. In contrast, tension-sided injuries located at the superolateral femoral neck are associated with a higher risk of displacement and often necessitate surgical intervention to prevent complications. Additionally, MRI is utilized to detect radiographically occult extensions of intertrochanteric or cervical fractures, particularly in cases involving known greater trochanteric fractures. This advanced imaging technique allows for more accurate assessment and treatment planning in complex fracture scenarios [38].
Multiple surgical and MRI-based classifications of labral tears exist; however, these classification systems exhibit poor agreement with one another. Discrepancies between the two approaches can complicate diagnosis and treatment planning, as each system may highlight different aspects of tear characteristics or location. This lack of consensus underscores the need for a more standardized and reliable classification system to enhance clinical decision-making and improve patient outcomes [39, 40].
On MRI, labral tears and detachments typically appear as linear hyperintense signals in fluid-sensitive sequences. MRA is considered the gold standard for evaluating the labrum, offering superior sensitivity and accuracy for detecting labral tears and detachments. The enhanced contrast provided by MRA allows for a clearer delineation of the labral structure, making it the most reliable imaging modality for assessing these injuries [36, 41]. On MRA, intrasubstance tears are characterized by contrast material within the labral substance, indicating disruption of labral tissue. In contrast, labral detachment is identified by the presence of contrast at the acetabular-labral interface, which signifies the separation of the labrum from the acetabulum. This distinct finding is a key diagnostic feature of labral detachment on MRA and provides valuable information for treatment planning [36].
Recent advances in medical imaging technologies
Three-dimensional (3D) imaging
In 2018, Professor Merloz summarized three decades of experience in image-guided orthopedic surgery, stating: “This recent, fascinating, and international journey, led by French teams at the forefront, is the result of collaboration across multiple scientific disciplines, including mathematics, computer science, radiology, physics, and medicine. Pre- or intraoperative imaging equips the surgeon with tools to visualize and interpret crucial information, facilitating the performance of the planned procedure with appropriate instruments, such as navigation, robotics, or personalized templates. The future of surgical navigation is influenced by the growing utilization of 3D intraoperative imaging and augmented reality technologies. This shift is already taking place, as evidenced by the publication of 2 688 articles in the past decade, which accounts for half of all publications on the subject [42].
The use of multislice CT (MSCT) in the operating room is limited by several factors. The equipment itself is large and not easily accommodated in surgical suites, and it is also a significant source of radiation. This restricts the types of procedures that can be performed in the CT room to simpler ones, such as biopsies or percutaneous procedures with linear trajectories, which are less invasive and impose fewer anesthetic challenges. More complex surgeries, such as those requiring fracture reduction or open implant insertion, are not feasible with MSCT in the operating room. However, MSCT provides very high-resolution images, which are crucial for procedures in sensitive body areas, such as neurosurgery. To address these limitations, newer MSCT units, such as AIRO® (Brainlab) and BodyTom (Samsung), have been developed specifically for operating rooms, making them more suitable for surgical settings. Cone-beam CT (CBCT) systems are advanced fluoroscopy units equipped with scintillation counters that enable the reconstruction of 3D images in the DICOM and Communications in Medicine format. Initially developed for use in dentistry, CBCT technology has since been adapted for various medical applications due to its ability to produce high-resolution images with lower radiation doses than traditional CT scanners. The compact nature of CBCT systems makes them particularly suitable for use in operating rooms, offering real-time imaging and facilitating more precise procedures [43, 44]. MSCT can be optimized for low-dose imaging, making it suitable for repeated use in CT-guided biopsies. However, CBCT has some limitations. The examination field is smaller due to the size of the detector, which provides less information about the surrounding soft tissues [45]. Additionally, this technology is more susceptible to noise from ray dispersion, reducing the contrast resolution. Truncation artifacts may also occur, and Hounsfield units cannot be used to characterize pixel density as in traditional CT. Despite these drawbacks, CBCT is particularly effective in orthopedic surgery, offering highly detailed images of bone structures. Early analog CBCT systems had the additional limitation of image distortion caused by the conical source projection in a 2D plane, requiring a calibration target and limiting the available working space. Modern flat-panel detector (FPD) technology has largely addressed this issue by digitizing images at the source, eliminating the need for a target. Modern FPD systems also avoid magnetic field distortion and provide a more extensive operating field, improving radiographic coverage and image resolution. For example, the Philips Veradius Unity mobile C-arm uses a 27×27-cm2 detector with 184-µm resolution, offering enhanced imaging capabilities while reducing radiation exposure to staff and patients. Nevertheless, CBCT, similar to MSCT, is susceptible to motion artifacts during image acquisition. Both technologies require patients to hold their breath, which can be challenging during procedures under local anesthesia. The new Surgivisio® system (eCential) addresses this issue by incorporating an acquisition coordinate system that allows imaging to be conducted during normal breathing, further improving the practicality of CBCT for surgical procedures [45].
Dynamic imaging
Hip arthrography is a pivotal diagnostic tool in managing Legg-Calvé-Perthes disease, offering critical insights that aid in tailoring treatment strategies to each patient’s specific needs. By providing real-time imaging of the hip joint, arthrography enables precise assessment of the femoral head’s congruence, extent of joint containment, and condition of the articular cartilage. This detailed visualization is particularly valuable for evaluating the dynamic relationship between the femoral head and acetabulum, which is essential for determining the optimal course of treatment. Moreover, hip arthrography assists in identifying subtle joint abnormalities that may not be apparent in conventional imaging modalities, such as plain radiographs or MRI. This technique is especially useful during surgical interventions, where it helps guide procedures, such as femoral or pelvic osteotomies, to ensure proper joint alignment and containment. As such, hip arthrography is an indispensable tool in the personalized management of Legg-Calvé-Perthes disease, contributing to improved clinical outcomes and preserving hip function over the long term [46-48]. In children with Legg-Calvé-Perthes disease, hip arthrography is a valuable diagnostic tool that provides dynamic imaging to evaluate critical aspects of joint morphology and function. This technique allows for detailed assessment of the congruency and containment of the femoral head within the acetabulum, which is pivotal in determining the stability and alignment of the hip joint. Additionally, hip arthrography is instrumental in excluding the presence of “hinge” abduction, a condition in which the lateral portion of the femoral head impinges on the acetabular rim during abduction, leading to restricted motion and joint incongruence. Identifying this abnormality is crucial because it often influences the choice of treatment, particularly when surgical interventions, such as femoral or pelvic osteotomies are being considered. Hip arthrography enhances the precision of treatment planning by offering real-time insights into hip biomechanics, ensuring that therapeutic strategies are customized to optimize joint function and long-term outcomes in patients with Legg-Calvé-Perthes disease [49]. Hinge abduction is a condition in which the femoral head cannot shift medially during abduction. This restriction occurs due to pronounced flattening of the femoral head, causing it to become obstructed by the lateral edge of the acetabulum, thereby limiting proper joint movement [48]. The primary goal of the management of Legg-Calvé-Perthes disease is to improve joint containment and congruity. Hip arthrography is crucial in achieving this by providing detailed information that helps identify patients who may benefit from surgical interventions such as periacetabular and intertrochanteric osteotomies. The insights gained from arthrography enable clinicians to make informed decisions about the suitability and timing of these procedures, optimize outcomes, and preserve hip function [49-51]. Identifying hinge abduction is crucial because addressing this condition is essential for ensuring proper development of the femoral head. Achieving this requires preserving adequate contact between the femoral head and the anatomically normal acetabulum. In numerous cases, this goal can be successfully attained through well-planned surgical interventions, which help restore joint congruence and promote optimal hip development [48]. Arthrography also facilitates the assessment of joint congruity during treatment strategies involving immobilization to promote epiphyseal healing. This imaging technique provides valuable insights into the alignment and stability of the joint, ensuring that immobilization effectively supports the healing process and maintains proper anatomical relationships [51].
MRI is a vital diagnostic modality for detecting early-stage or radiographically occult Legg-Calvé-Perthes disease because it provides detailed visualization of the extent of epiphyseal involvement. Its ability to capture soft tissue and bone marrow changes with high resolution makes it indispensable for early identification and comprehensive evaluation of the disease [52]. Dynamic hip examination using MRI is constrained by the enclosed design of conventional MRI systems, which limits its ability to evaluate joint function in various positions. However, the increasing availability of open-configuration MRI systems has addressed this limitation, enabling dynamic imaging of joints in multiple positions. This advancement allows for a more comprehensive assessment of joint mechanics and alignment, enhancing diagnostic and treatment planning capabilities.
Low-dose imaging systems
The incidence of hip preservation surgery among adolescents and young adults has steadily increased in recent years. Achieving favorable clinical outcomes and reducing the likelihood of revision surgeries depends heavily on comprehensive correction of the underlying osseous pathomorphology. Precise and thorough surgical intervention aimed at addressing bony deformities is essential for restoring joint functionality, alleviating pain, and preventing long-term complications, such as early onset osteoarthritis or joint instability. This underscores the importance of meticulous preoperative planning and advanced surgical techniques tailored to each patient’s unique anatomical and biomechanical challenges [53-57]. Plain radiographs have notable limitations in accurately capturing the 3D characteristics of hip morphology. These limitations become particularly evident when attempting to precisely localize and evaluate cam deformities or acetabular morphology, especially in the context of variable pelvic tilt and rotation. While some surgeons continue to rely exclusively on plain radiographs in combination with intraoperative dynamic fluoroscopic assessments to evaluate bony morphology, there is a growing shift toward incorporating CT scans into the preoperative assessment process. CT imaging provides superior visualization and quantitative data, offering advanced 3D reconstructions that enhance our understanding of the complex anatomy of the pelvis, acetabulum, and femur. This level of detail is invaluable for identifying subtle deformities and obtaining a more accurate assessment of the underlying pathomorphology. Additionally, CT scans help resolve potential diagnostic ambiguities associated with plain radiographs, such as false-positive findings, including the crossover sign or posterior wall sign. They also enable clinicians to account for and adjust pelvic tilt or rotation changes, which can significantly affect the accuracy of conventional radiographic interpretation. As such, integrating CT imaging into preoperative planning is becoming an increasingly valuable tool for achieving precise diagnoses and optimizing surgical outcomes in hip preservation surgery [58]. Despite the enhanced information provided by CT scans, one of the primary drawbacks is increased radiation exposure, which poses particular concern for hip-preservation surgeons treating adolescents and young adults who are more sensitive to radiation. However, recent technological advancements and the development of refined scanning protocols have led to substantial reductions in the radiation doses during these scans. Low-dose CT imaging protocols have been successfully implemented across pediatric and adult populations, facilitating various diagnostic and surgical procedures with minimal risks. Nevertheless, the application of low-dose CT specifically for preoperative planning in hip preservation surgeries remains insufficiently established. Although the potential benefits of reducing radiation exposure are clear, there is a need for further research and clinical validation to determine the efficacy and safety of low-dose hip CT scans in surgical planning. This includes exploring whether the reduced radiation dose still provides the necessary detail and accuracy for optimal preoperative assessment, especially in the delicate patient population requiring hip preservation procedures. Therefore, further studies are essential to evaluate the feasibility and reliability of low-dose CT as a standard tool for hip preservation surgical planning [59-64].
Expected effects of artificial intelligence (AI) in hip fracture diagnosis
The swift identification of non-displaced hip fractures can be challenging for clinicians and may necessitate additional imaging techniques, such as radiography, bone scans, CT, or MRI. However, these supplementary tests may not be readily accessible at all healthcare facilities. Moreover, factors, such as bone demineralization and overlying soft tissues can complicate the accurate diagnosis of hip fractures [65]. Delayed diagnosis and treatment of hip fractures can result in complications, such as mal:union:, osteonecrosis, and arthritis [66]. Additionally, with the increasing number of imaging and radiological examinations, radiology departments often cannot report all acquired radiographs promptly [67]. As a result, several studies have been conducted on using machine learning to detect hip fractures [65, 66, 68-72]. Early diagnosis of hip fractures using AI algorithms in clinical practice could help reduce medical costs, enable more effective preventive measures, and improve the overall quality of healthcare [71]. AI-driven early diagnosis of hip fractures also enhances resource allocation, reduces unnecessary consultations, and accelerates patient disposition. This allows physicians to focus on more complex tasks in high-volume clinical settings. However, there is a lack of sufficient reports on the effectiveness of AI algorithms for early diagnosis of hip fractures, suggesting that further research is necessary.
AI versus human
The results of the studies included in the analysis showed that the accuracy of hip fracture diagnosis using AI algorithms was over 90%, with the exception of Beyaz et al. Additionally, the area under the curve (AUC) for fracture diagnosis consistently exceeded 0.9, indicating a very high diagnostic performance [70]. In a comparative study between AI and human diagnosis of hip fractures, the diagnostic accuracy of AI was found to be higher. For instance, Urakawa et al. developed an AI model that detected intertrochanteric fractures with an accuracy of 95.5% and AUC of 0.984, demonstrating the high performance of AI in identifying these fractures [73]. The AI model developed by Urakawa et al. outperformed the human diagnostic accuracy of 92.2%, with an AUC of 0.969. Adams et al. reported a conventional neural network model for diagnosing femoral neck fractures, achieving an accuracy range of 88.1% to 94.4%. This highlights the potential of AI to surpass human performance in certain diagnostic contexts [74]. These values are also comparable to the diagnostic accuracies of experts and residents, which were 93.5% and 92.9%, respectively. However, in studies conducted by Cheng et al. [71] and Sato et al. [75], the human diagnostic accuracy was lower than that of the AI algorithm. This further underscores the potential of AI to achieve higher diagnostic performance in detecting hip fractures [71]. Nevertheless, whether AI can fully replace humans in hip fracture diagnosis remains uncertain. Bae et al. utilized AI to diagnose femoral neck fractures after training the algorithm with 4189 images, achieving a diagnostic accuracy of 97.1%. However, they noted that detecting non-displaced fractures of the femoral neck remains challenging despite the high overall diagnostic accuracy of the AI model. This highlights a limitation of current AI systems in addressing specific fracture types, suggesting that while AI can be highly accurate, human expertise may still be necessary for certain cases [72]. This suggests that AI may have limitations in cases where it has not been adequately trained or lacks sufficient learning. Additionally, since the AI systems examined in the study are not integrated with other clinical information, they cannot yet replicate the clinical judgment of a human physician, such as the ability to suspect occult fractures based on a patient’s overall condition. Mawatari et al. also argued that since the AUC values for AI-assisted expert diagnoses were higher than those for the AI algorithm alone, a valid diagnosis could not be obtained from radiographs alone. This highlights that the effectiveness of AI diagnosis is still heavily influenced by the quality and scope of the AI algorithm [65]. Therefore, AI algorithms cannot entirely replace human intelligence in the clinical environment. However, they can complement and enhance physicians’ capabilities and knowledge. A potential concern is the increasing reliance on AI for hip fracture detection, as it may reduce the need for doctors to make their clinical judgments. This reliance can stem from the difficulty and time constraints clinicians face in synthesizing the results of various examinations performed in direct patient interactions. Balancing AI assistance with clinical expertise remains essential for comprehensive patient care [71]. To address this issue, Cheng et al. developed a system in which the areas identified by AI for hip fracture detection were highlighted and displayed, allowing physicians to review the results of the AI algorithm and make the final clinical judgment. This approach enables clinicians to integrate AI findings with their expertise, ensuring that the decision-making process remains collaborative and informed by the algorithm and physician’s assessment [71]. With advancements in technology, AI algorithms are expected to continue evolving, leading to increased reliance on AI. However, this growing dependence on AI may present challenges in balancing between human judgment and algorithmic assistance. Further research is essential to explore solutions to these challenges, ensuring that AI can complement, rather than replace, human expertise in clinical decision-making.
AI deep learning for hip fracture
Because deep learning algorithms in AI automatically and adaptively learn features from data, the quality and quantity of datasets used for training are critical. Large, clean, and well-labeled datasets ensure that AI models can effectively learn and generalize from diverse clinical scenarios, ultimately leading to more accurate and reliable performance in real-world applications [70]. The accuracy of AI in detecting hip fractures is significantly influenced by the number of images used for training the model. A larger dataset allows the AI system to learn from various cases, improving its ability to generalize and detect fractures across different patient populations and imaging conditions. As the number of images increases, the AI’s performance typically improves because it becomes better equipped to identify subtle patterns and variations in the data indicative of hip fractures [75]. Mutasa et al. created 9063 augmented images by combining 737 hip fracture images with 326 normal images from a dataset of 550 patients. Beyaz et al. also generated 2 106 augmented images from 234 radiographs of 65 patients. By augmenting the data, these studies aimed to enhance the AI models’ ability to learn from a more diverse set of images, thereby improving the accuracy and robustness of fracture detection algorithms. This augmentation process helps the model to generalize better across different patient demographics and imaging conditions [69, 70]. Yu et al. reported that detecting a distinct fracture line or cortical angular deformity in femoral neck fractures is relatively straightforward using a single radiographic view. However, a larger sample size is necessary for accurate detection due to the wide spectrum of fracture morphologies for intertrochanteric fractures, which often involve complex and multiple fracture lines. This suggests that although some fracture types are easier to identify with a single radiograph, more intricate fractures require a broader dataset to enhance diagnostic accuracy, particularly when using AI algorithms for detection [68]. Additionally, factors, such as soft-tissue shading and variations in femur alignment, can impact the ability of AI to detect fractures accurately. Soft tissue shadows can obscure fracture lines, making it more difficult for AI to distinguish between fractures and surrounding structures. Similarly, variations in femoral alignment, such as differences in positioning or angle during imaging, can alter the appearance of fractures and potentially lead to false positives or negatives in AI detection. These challenges highlight the need for careful image acquisition and consideration of such factors when developing and using AI models for hip fracture diagnosis [76]. Additionally, factors, such as soft-tissue shading and variations in femur alignment, can impact the ability of AI to detect fractures accurately. Soft tissue shadows can obscure fracture lines, making it more difficult for AI to distinguish between fractures and surrounding structures. Similarly, variations in femur alignment, such as differences in positioning or angle during imaging, can alter the appearance of fractures and potentially lead to false positives or negatives in AI detection. These challenges highlight the need for careful image acquisition and consideration of such factors when developing and using AI models for hip fracture diagnosis [66]. In contrast, Yoon et al. reported that the use of both CT images and radiographs for the classification of intertrochanteric fractures significantly reduced the time required for fracture classification. This approach also assisted in planning more accurate surgical procedures. By providing more detailed and comprehensive imaging data, CT scans helped clarify fracture patterns, enabling surgeons to make better-informed decisions regarding the appropriate treatment strategy. This integration of multiple imaging modalities highlights the value of combining radiographs with advanced techniques, such as CT, to improve the efficiency and accuracy of hip fracture management [77]. Mawatari et al. used MRI and CT for hip fracture detection [65]. However, this approach has the disadvantage of incurring additional costs and the challenge of obtaining a normal lateral view of the hip in some cases. Despite these limitations, AI offers significant potential for diagnosing and classifying diseases because it rapidly processes large volumes of patient data. By efficiently analyzing complex imaging data, AI can enhance diagnostic accuracy, reduce the time spent on classification tasks, and support clinical decision-making, while minimizing the dependency on costly and difficult-to-obtain imaging views [78]. The usefulness of AI is being actively studied in trauma prediction, a field characterized by significant individual variability in the number and severity of injuries. This variability arises from the complex interaction of various external and internal factors, such as the mechanism of injury, patient demographics, and pre-existing health conditions. By processing vast amounts of data, AI algorithms can identify patterns and predict outcomes more accurately than traditional methods, helping anticipate the trauma’s extent and guide clinical interventions. This potential for personalized trauma care can significantly improve patient outcomes and optimize resource allocation in trauma settings [79].
Innovative molecular imaging techniques
Positron emission tomography (PET)/CT and PET/MRI
Since the 1960s, radionuclide bone scanning h::as char::acterized a wide range of bone pathologies. In addition to their ability to detect bone metastases from various tumors well before other radiographic modalities of that era, bone scans gained recognition for their superior sensitivity in identifying occult fractures, stress fractures, osteoid osteomas, and other musculoskeletal conditions of interest to orthopedists. While some nuclear medicine practices developed significant expertise in diagnosing soft tissue injuries, most studies have focused primarily on evaluating bony pathology. Therefore, bone scans has become an essential diagnostic tool in orthopedics, particularly for conditions that are difficult to detect using conventional radiography [80]. Several factors have contributed to the gradual reduction in the use of bone scanning in orthopedics. Many injuries requiring surgical intervention primarily involve soft tissues around the joints. MRI has advanced significantly over the past three decades, providing clear delineation of most soft tissue pathologies. While MRI’s specificity for bone conditions is somewhat limited, it can surpass bone scanning in certain cases, particularly in older patients with acute hip trauma, because it often detects fractures days before a bone scan shows positive results [81]. MRI compensates for its limited specificity for bone pathology by providing exceptional anatomical details. In many nuclear medicine practices, bone scanning in orthopedics has been primarily reserved for patients with stress injuries or certain conditions in which other imaging modalities have failed to diagnose definitively. The sensitivity of bone scanning can be enhanced by incorporating single-photon emission computed tomography; however, the absence of detailed anatomical information often makes it challenging to interpret results confidently [82].
PET has historically been underused in musculoskeletal imaging due to several factors, including the high effectiveness of musculoskeletal MRI, the limited spatial resolution of PET, and the lack of reimbursement for such studies. However, with advancements in PET/CT and PET/MRI technology over the past decade, along with a deeper understanding of the pathophysiology of musculoskeletal diseases, PET is emerging as a promising primary or complementary imaging modality for managing rheumatologic and orthopedic conditions. The low metabolic activity of bones and tendons can be beneficial and challenging for PET/CT evaluation. However, these structures’ minimal physiological background activity enhances the target-to-background ratio when imaging disease processes, which is a significant advantage. In contrast, non-neoplastic, non-inflammatory musculoskeletal conditions, such as traumatic injuries, tendon, and ligament abnormalities, can exhibit low metabolic activity, making them less detectable by PET [83, 84].
PET/CT versus PET/MRI
PET/MRI, as a hybrid imaging modality, offers several advantages over PET/CT, including reduced radiation exposure for patients and superior soft-tissue contrast. This higher soft-tissue resolution is particularly beneficial for the diagnostic assessment of tendinous and ligamentous pathological conditions, where detailed visualization of soft tissues is essential [85]. Most importantly, PET/MRI outperforms PET/CT in terms of superior co-registration of PET data with MRI, and enhanced motion correction based on MRI. This combination leads to more accurate and reliable imaging, particularly in assessing dynamic musculoskeletal structures where motion artifacts can be problematic [85]. PET/MRI has been regarded as a comprehensive solution for the diagnostic management of patients with musculoskeletal disorders, as it allows for the simultaneous acquisition of multisequence, multiparametric MRI, and whole-body PET imaging. This makes it particularly efficient for patients requiring extensive MRI workups. However, PET/CT still offers superior attenuation correction, is more widely accessible, and is less expensive. A key limitation of PET/MRI is that the standardized uptake values derived from PET/MRI can be scanner-dependent, complicating direct comparisons between PET/CT and PET/MRI data [83, 85].
Studies have demonstrated that in the assessment of suspected knee and hip prosthetic infections, FDG PET offers superior accuracy compared to the combination of bone marrow and leukocyte scintigraphy, with accuracy rates ranging from 80% to 98%, versus 25% to 96% for the latter approach [86]. The sensitivity of combined bone marrow and leukocyte scintigraphy is 30%–35%. However, studies have shown that FDG PET, when used to evaluate patients with suspected hip prosthetic infections, demonstrates similar sensitivity but slightly lower specificity than combined bone marrow and leukocyte scintigraphy [87]. It has also been shown that PET provides no additional benefit over combined bone marrow and leukocyte scintigraphy when evaluating total knee arthroplasties [88].
Despite conflicting data regarding the diagnostic performance of FDG PET in periprosthetic infections compared to imaging with single-photon emitters, PET presents several advantages. These include time savings (as it does not require dual-image acquisition, such as dual-tracer techniques), higher spatial resolution, and a better cost and safety profile compared to the complexity and risks associated with using labeled white blood cells, which involve handling blood products. As a result, FDG PET/CT is a suitable diagnostic imaging method for patients with suspected hardware infections [89].
PET/CT has been studied for the management of patients with suspected implant-related infections of the tibia after osteosynthesis. FDG PET/CT assisted in clinical decision-making by distinguishing between infected non:union:, aseptic non:union:, soft-tissue infection, and chronic osteomyelitis. The overall sensitivity of FDG PET/CT for identifying osseous infections was 85.7%, with a specificity of 100%. This highlights the potential of FDG PET/CT to accurately diagnose implant-related infections and guide treatment decisions [89].
The role of nanotechnology in early detection
Nanotechnology is the science of designing, manipulating, and engineering materials at molecular and atomic scales. In bone regeneration, this technology is used to create new bone tissue or enhance the healing of existing bone tissue by utilizing materials that stimulate and support bone growth. Owing to their small size and unique properties, nanomaterials can be integrated into scaffolds, coatings, or drug delivery systems to improve bone regeneration, promote cell growth, and accelerate the healing process in fractures or bone defects [90, 91]. Nanotechnology is increasingly employed in bone regeneration to develop more precise, effective, and targeted materials that enhance bone growth. By manipulating materials at the nanoscale, scientists can create scaffolds, coatings, and drug delivery systems that are better suited to mimic the natural bone environment. These nanomaterials improve cell adhesion, proliferation, and differentiation, thereby accelerating bone healing and regeneration. Additionally, their small size allows for enhanced bioactivity, better integration with surrounding tissues, and more controlled delivery of therapeutic agents, ultimately leading to more successful bone repair and regeneration [92]. For example, researchers are investigating the use of nanoparticles to deliver drugs or other therapeutic molecules directly to areas in need of bone regeneration, enhancing the treatment’s effectiveness. These nanoparticles can be engineered to target specific sites, such as bone fractures or areas of degeneration, allowing for more precise and localized treatment. By controlling the release of growth factors, osteoinductive molecules, or other regenerative agents, nanoparticles ensure that these compounds are delivered at optimal concentrations at the right time, maximizing their therapeutic potential and minimizing side effects. This targeted approach holds great promise for improving the outcomes of bone healing and regeneration therapies [92]. Nanoparticles can also be utilized to create scaffolds that replicate the natural structure of bones, providing a framework to guide and support new bone growth. These nanostructured scaffolds can enhance cell adhesion, proliferation, and differentiation and promote more efficient bone regeneration. Moreover, advancements in 3D printing technology, incorporating nanoscale materials, enable the fabrication of highly precise, customized implants tailored to individual patients’ anatomical needs. These implants can be designed to match the patient’s bone structure, optimize the healing process, and ensure better integration with the surrounding tissue. Together, nanotechnology and 3D printing innovations hold great potential for revolutionizing bone regeneration therapies, offering more effective and personalized treatments for bone-related injuries and conditions [90]. Bone weakening and dysfunction are significant health concerns, particularly in osteoporosis, fractures, and age-related bone degeneration. These challenges have prompted nanotechnologists to focus on the potential of nanotechnology in addressing bone-related issues, marking it a crucial area of research in the intersection of nanotechnology and medicine. Studies are increasingly exploring how nanotechnology can aid bone formation, structuring, and repair. Nanotechnology offers the potential to develop advanced biomaterials that can mimic the natural bone structure at the nanoscale, enhance bone strength, and promote regeneration. Researchers are investigating the use of nanoparticles to deliver therapeutic agents directly to bone tissue, stimulate bone formation, and improve bone density. Nanostructured scaffolds are being developed to provide an ideal environment for bone cell growth, ultimately supporting the formation of new bone tissue and enhancing the healing process after fractures or surgeries. These advancements can revolutionize treatments for conditions that weaken bones and promote more effective strategies for bone regeneration and repair [90]. Scientists are working on developing bone graft substitutes using nanostructured materials that closely mimic the natural properties of bones. These materials are biocompatible, allowing them to be seamlessly integrated into the body without triggering an immune response. If these studies succeed, they have the potential to revolutionize regenerative medicine by offering more effective solutions for repairing damaged bones and tissues. Creating bone grafts that promote natural bone regeneration can significantly improve the healing process of fractures, bone defects, and conditions, such as osteoporosis, offering a promising alternative to traditional bone repair methods. The development of nanostructured grafts is a step toward advanced regenerative technologies, where the material serves as a scaffold for bone growth and actively supports cellular activity, promoting healing and tissue regeneration. This could lead to improved outcomes in treating bone injuries, potentially reducing the need for long recovery times and offering patients more effective treatment options for broken or damaged bone and muscle fragments [93]. Principal investigations into biomineralization are focused on reducing the particle size of bone materials to enhance their interaction with collagen fibers. This approach aims to replicate the natural process by which bones mineralize and incorporate minerals into a collagen matrix to create a strong and functional composite structure. By reducing the particle size of bone materials, researchers can increase the surface area and improve the bonding efficiency with collagen fibers, allowing for better integration and stability within the bone matrix. This process also seeks to mimic the crystalline properties of bones, which are essential for their strength and resilience. Coupling fine mineral particles with collagen fibers can improve the material’s mechanical properties, making it more effective in supporting bone regeneration and repair. Additionally, this method can potentially enhance the material’s bioactivity, encouraging cellular activity that promotes bone healing and regeneration. Ultimately, these investigations aim to develop advanced bone substitutes that more closely resemble natural bone tissue in terms of structure and function. This can lead to better outcomes in regenerative medicine, offering more effective treatments for bone fractures, defects, and degenerative conditions [92]. The aim is to develop a composition that effectively penetrates damaged bone regions and possesses tailored mechanical properties to transform the field of osteology and advance bone tissue engineering [92]. Similar research is underway to develop artificial joints with nanoscale collagen-mimicking coatings for the knee and hip, which help stabilize the bone-formation process by osteoblasts [94, 95]. In summary, the application of nanotechnology in bone regeneration offers significant potential to enhance bone repair and regeneration outcomes, leading to faster healing, stronger bones, and fewer complications.
Conclusion
In conclusion, recent advancements in imaging technologies have offered significant improvements in the diagnosis of hip disorders, enabling more accurate, timely, and efficient assessments. Conventional imaging techniques remain vital, but emerging technologies, such as AI, 3D and dynamic imaging, and molecular imaging, are expanding diagnostic capabilities. AI has shown promising potential for the rapid and accurate diagnosis of hip fractures, particularly non-displaced fractures, which can be difficult for clinicians to detect. Additionally, the application of PET/CT and PET/MRI to assess metabolic changes and the role of nanotechnology in early detection represent groundbreaking steps. However, challenges related to cost, accessibility, and integration into clinical workflows must be addressed for these technologies to reach their full potential. Continued research and innovation are necessary to optimize these tools for routine clinical use, ultimately improving patient outcomes in managing hip disorders.
Limitations
In addition to the advancements above, each imaging modality has its own set of challenges, advantages, and limitations. These factors are outlined and discussed in detail (Figure 3).
Ethical Considerations
Compliance with ethical guidelines
There were no ethical considerations to be considered in this research.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Conceptualization: Karim Pisoudeh, and Khatere Mokhtari; Methodology: Siamak Kazemi;Data collection: Karim Pisoudeh, Khatere Mokhtari, and Siamak Kazemi; Data analysis: Khatere Mokhtari and Siamak Kazemi; Investigation, and writing: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to express their gratitude to the Department of Orthopedics, Bone and Joint Reconstruction Research Center, School of Medicine, Shafayahyaeian Hospital, Iran University of Medical Sciences, Tehran, Iran, for their invaluable support in conducting this research. Additionally, we would like to thank the Department of Cellular and Molecular Biology and Microbiology, Faculty of Biological Science and Technology, University of Isfahan, Isfahan, Iran, for their assistance in providing the necessary expertise.
References
- Picavet HS, Schouten JS. Musculoskeletal pain in the Netherlands: Prevalences, consequences and risk groups, the DMC(3)-study. Pain. 2003; 102(1-2):167-78. [DOI:10.1016/s0304-3959(02)00372-x] [PMID]
- Christmas C, Crespo CJ, Franckowiak SC, Bathon JM, Bartlett SJ, Andersen RE. How common is hip pain among older adults. J Fam Pract. 2002; 51(4):346-8. [Link]
- Wilson JJ, Furukawa M. Evaluation of the patient with hip pain. Am Fam Physician. 2014; 89(1):27-34. [Link]
- Chang A,Breeland G, Black AC, Hubbard JB. Anatomy, bony pelvis and lower limb: Femur. Treasure Island: StatPearls; 2023. [Link]
- Alzaharani A, Bali K, Gudena R, Railton P, Ponjevic D, Matyas JR, et al. The innervation of the human acetabular labrum and hip joint: An anatomic study. BMC Musculoskelet Disord. 2014; 15:41. [DOI:10.1186/1471-2474-15-41] [PMID] [PMCID]
- Kazakia GJ, Majumdar S. New imaging technologies in the diagnosis of osteoporosis. Rev Endocr Metab Disord. 2006; 7(1-2):67-74. [DOI:10.1007/s11154-006-9004-2] [PMID]
- Angelopoulos C, Aghaloo T. Imaging technology in implant diagnosis. Dent Clin North Am. 2011; 55(1):141-58. [DOI:10.1016/j.cden.2010.08.001] [PMID] [PMCID]
- Hussain S, Mubeen I, Ullah N, Shah SSUD, Khan BA, Zahoor M, et al. Modern diagnostic imaging technique applications and risk factors in the medical field: A review. Biomed Res Int. 2022; 2022:5164970. [DOI:10.1155/2022/5164970] [PMID] [PMCID]
- Conn KS, Clarke MT, Hallett JP. A simple guide to determine the magnification of radiographs and to improve the accuracy of preoperative templating. J Bone Joint Surg Br Vol. 2002; 84(2):269-72. [DOI:10.1302/0301-620X.84B2.0840269]
- Wimsey S, Pickard R, Shaw G. Accurate scaling of digital radiographs of the pelvis. A prospective trial of two methods. J Bone Joint Surg Br. 2006; 88(11):1508-12. [DOI:10.1302/0301-620X.88B11.18017] [PMID]
- The B, Diercks RL, Stewart RE, van Ooijen PM, van Horn JR. Digital correction of magnification in pelvic x rays for preoperative planning of hip joint replacements: Theoretical development and clinical results of a new protocol. Med Phys. 2005; 32(8):2580-9. [DOI:10.1118/1.1984293] [PMID]
- Novosad J, Cheriet F, Petit Y, Labelle H. Three-dimensional (3-D) reconstruction of the spine from a single X-ray image and prior vertebra models. IEEE Trans Biomed Eng. 2004; 51(9):1628-39. [DOI:10.1109/TBME.2004.827537] [PMID]
- Cheriet F, Laporte C, Kadoury S, Labelle H, Dansereau J. A novel system for thE 3-D reconstruction of the human spine and rib cage from biplanar X-ray images. IEEE Trans Biomed Eng. 2007; 54(7):1356-8. [DOI:10.1109/TBME.2006.889205] [PMID]
- Mitton D, Zhao K, Bertrand S, Zhao C, Laporte S, Yang C, et al. 3D reconstruction of the ribs from lateral and frontal X-rays in comparison to 3D CT-scan reconstruction. J Biomech. 2008; 41(3):706-10. [DOI:10.1016/j.jbiomech.2007.09.034] [PMID]
- Hounsfield GN. Computerized transverse axial scanning (tomography). 1. Description of system. Br J Radiol. 1973; 46(552):1016-22. [DOI:10.1259/0007-1285-46-552-1016] [PMID]
- Hounsfield GN. Picture quality of computed tomography. AJR Am J Roentgenol. 1976; 127(1):3-9. [DOI:10.2214/ajr.127.1.3] [PMID]
- O'sullivan GS, Goodman SB, Jones HH. Computerized tomographic evaluation of acetabular anatomy. Clin Orthop Relat Res. 1992; 277:175-81. [DOI:10.1097/00003086-199204000-00021]
- Keene GC, Hone MR, Sage MR. Atlas fracture: Demonstration using computerized tomography. A case report. JBJS. 1978; 60(8):1106-7. [DOI:10.2106/00004623-197860080-00017]
- Ter-Pogossiam MM. The challenge of computed tomography. AJR Am J Roentgenol. 1976; 127(1):1-2. [DOI:10.2214/ajr.127.1.1] [PMID]
- Watson RC. CT scan--its use and abuse. CA Cancer J Clin. 1978; 28(2):100-3. [DOI:10.3322/canjclin.28.2.100] [PMID]
- Hubbard LF, Mcdermott JH, Garrett G. Computed axial tomography in musculoskeletal trauma. J Trauma Acute Care Surg. 1982; 22(5):388-94. [DOI:10.1097/00005373-198205000-00007] [PMID]
- Rowe CR, Lowell JD. Prognosis of fractures of the acetabulum. JBJS. 1961; 43(1):30-92. [DOI:10.2106/00004623-196143010-00002]
- Naidich DP, Freedman MT, Bowerman JW, Siegelman SS. Ten section approach to computed tomography of the pelvis. Skeletal Radiol. 1980; 5(4):213-7. [DOI:10.1007/BF00580592] [PMID]
- Ladd LM, Blankenbaker DG, Davis KW, Keene JS. MRI of the hip: Important injuries of the adult athlete. Curr Radiol Rep. 2014; 2:1-9. [DOI:10.1007/s40134-014-0051-2]
- Schmaranzer F, Todorski IA, Lerch TD, Schwab J, Cullmann-Bastian J, Tannast M. Intra-articular lesions: Imaging and surgical correlation. Semin Musculoskelet Radiol. 2017; 21(05):487-506. [DOI:10.1055/s-0037-1606133]
- Chang CY, Huang AJ. MR imaging of normal hip anatomy. Magn Reson Imaging Clin N Am. 2013; 21(1):1-19. [DOI:10.1016/j.mric.2012.08.006] [PMID]
- Naraghi A, White LM. MRI of labral and chondral lesions of the hip. AJR Am J Roentgenol. 2015; 205(3):479-90. [DOI:10.2214/AJR.14.12581] [PMID]
- Blankenbaker DG, Tuite MJ. The painful hip: New concepts. Skeletal Radiol. 2006; 35(6):352-70. [DOI:10.1007/s00256-006-0105-5] [PMID]
- Agten CA, Sutter R, Buck FM, Pfirrmann CW. Hip imaging in athletes: Sports imaging series. Radiology. 2016; 280(2):351-69. [DOI:10.1148/radiol.2016151348] [PMID]
- Ilizaliturri VM Jr, Byrd JW, Sampson TG, Guanche CA, Philippon MJ, Kelly BT, et al. A geographic zone method to describe intra-articular pathology in hip arthroscopy: Cadaveric study and preliminary report. Arthroscopy. 2008; 24(5):534-9. [DOI:10.1016/j.arthro.2007.11.019] [PMID]
- Sutter R, Zubler V, Hoffmann A, Mamisch-Saupe N, Dora C, Kalberer F, et al. Hip MRI: How useful is intraarticular contrast material for evaluating surgically proven lesions of the labrum and articular cartilage? AJR Am J Roentgenol. 2014; 202(1):160-9. [DOI:10.2214/AJR.12.10266] [PMID]
- Magee T. Comparison of 3.0-T MR vs 3.0-T MR arthrography of the hip for detection of acetabular labral tears and chondral defects in the same patient population. Br J Radiol. 2015; 88(1053):20140817. [DOI:10.1259/bjr.20140817] [PMID] [PMCID]
- Llopis E, Cerezal L, Kassarjian A, Higueras V, Fernandez E. Direct MR arthrography of the hip with leg traction: Feasibility for assessing articular cartilage. AJR Am J Roentgenol. 2008; 190(4):1124-8. [DOI:10.2214/AJR.07.2559] [PMID]
- Schmaranzer F, Klauser A, Kogler M, Henninger B, Forstner T, Reichkendler M, et al. Diagnostic performance of direct traction MR arthrography of the hip: Detection of chondral and labral lesions with arthroscopic comparison. Eur Radiol. 2015; 25(6):1721-30. [DOI:10.1007/s00330-015-3641-3] [PMID]
- Sheehan SE, Shyu JY, Weaver MJ, Sodickson AD, Khurana B. Proximal femoral fractures: What the orthopedic surgeon wants to know. Radiographics. 2015; 35(5):1563-84. [DOI:10.1148/rg.2015154017] [PMID]
- Teh J. Imaging the hip. Imaging. 2007; 19(3):234. [DOI:10.1259/imaging/48691706]
- Long MM, Stetts DM. Stress fractures of the femoral neck. Orthop Nurs. 1985; 4(3):69-71. [DOI:10.1097/00006416-198505000-00014] [PMID]
- Kim SJ, Ahn J, Kim HK, Kim JH. Is magnetic resonance imaging necessary in isolated greater trochanter fracture? A systemic review and pooled analysis. BMC Musculoskelet Disord. 2015; 16:395. [DOI:10.1186/s12891-015-0857-y] [PMID] [PMCID]
- Blankenbaker DG, De Smet AA, Keene JS, Fine JP. Classification and localization of acetabular labral tears. Skeletal Radiol. 2007; 36(5):391-7. [DOI:10.1007/s00256-006-0240-z] [PMID]
- Freedman BA, Potter BK, Dinauer PA, Giuliani JR, Kuklo TR, Murphy KP. Prognostic value of magnetic resonance arthrography for Czerny stage II and III acetabular labral tears. Arthroscopy. 2006; 22(7):742-7. [DOI:10.1016/j.arthro.2006.03.014] [PMID]
- Czerny C, Hofmann S, Urban M, Tschauner C, Neuhold A, Pretterklieber M, et al. MR arthrography of the adult acetabular capsular-labral complex: Correlation with surgery and anatomy. AJR Am J Roentgenol. 1999; 173(2):345-9. [DOI:10.2214/ajr.173.2.10430132] [PMID]
- Merloz P, Troccaz J, Cinquin P, Lavallée S. [La chirurgie orthopédique et traumatologique guidée par l’image. De la naissance à l’âge adulte (French)]. Un siècle d’innovations françaises en chirurgie orthopédique et traumatologique–1918-2018. 2018; 47-55. [Link]
- Arai Y, Tammisalo E, Iwai K, Hashimoto K, Shinoda K. Development of a compact computed tomographic apparatus for dental use. Dentomaxillofac Radiol. 1999; 28(4):245-8. [DOI:10.1038/sj/dmfr/4600448] [PMID]
- Kailash S. CBCT-Cone beam computed tomography. J Acad Dent Educ. 2014; 1(1):9-15. [DOI:10.18311/jade/2014/2423]
- Tonetti J, Boudissa M, Kerschbaumer G, Seurat O. Role of 3D intraoperative imaging in orthopedic and trauma surgery. Orthop Traumatol Surg Res. 2020; 106(1S):S19-25. [DOI:10.1016/j.otsr.2019.05.021] [PMID]
- Gershuni DH, Axer AN, Hendel DA. Arthrographic findings in Legg-Calvé-Perthes disease and transient synovitis of the hip. JBJS. 1978; 60(4):457-64. [DOI:10.2106/00004623-197860040-00005]
- Gallagher JM, Weiner DS, Cook AJ. When is arthrography indicated in legg-calvé-perthes disease? JBJS. 1983; 65(7):900-5. [DOI:10.2106/00004623-198365070-00004]
- Quain S, Catterall A. Hinge abduction of the hip. Diagnosis and treatment. J Bone Joint Surg Br. 1986; 68(1):61-4. [DOI:10.1302/0301-620X.68B1.3941142] [PMID]
- Catterall A. The natural history of perthes’disease. J Bone Joint Surg Br Vol. 1971; 53(1):37-53. [DOI:10.1302/0301-620X.53B1.37]
- Herring JA. The treatment of legg-calvé-perthes disease. A critical review of the literature. J Bone Joint Surg Am. 1994; 76(3):448-58. [DOI:10.2106/00004623-199403000-00017] [PMID]
- Weinstein S. Legg-calvé-perthes syndrome. In: Morrisy RT, Weistein SL, editors. Lovell and winter’s pediatrics orthopaedics, 5th edition. Philadelphia: Lippincott Williams and Wilkins; 2001. [Link]
- Bos CF, Bloem JL, Bloem RM. Sequential magnetic resonance imaging in Perthes' disease. J Bone Joint Surg Br. 1991; 73(2):219-24. [DOI:10.1302/0301-620X.73B2.2005143] [PMID]
- Clohisy JC, Nepple JJ, Larson CM, Zaltz I, Millis M; Academic Network of Conservation Hip Outcome Research (ANCHOR) Members. Persistent structural disease is the most common cause of repeat hip preservation surgery. Clin Orthop Relat Res. 2013; 471(12):3788-94. [DOI:10.1007/s11999-013-3218-x] [PMID] [PMCID]
- Bogunovic L, Gottlieb M, Pashos G, Baca G, Clohisy JC. Why do hip arthroscopy procedures fail? Clin Orthop Relat Res. 2013; 471(8):2523-9. [DOI:10.1007/s11999-013-3015-6] [PMID] [PMCID]
- Wenger DE, Kendell KR, Miner MR, Trousdale RT. Acetabular labral tears rarely occur in the absence of bony abnormalities. Clin Orthop Relat Res. 2004; (426):145-50. [DOI:10.1097/01.blo.0000136903.01368.20] [PMID]
- Heyworth BE, Shindle MK, Voos JE, Rudzki JR, Kelly BT. Radiologic and intraoperative findings in revision hip arthroscopy. Arthroscopy. 2007; 23(12):1295-302. [DOI:10.1016/j.arthro.2007.09.015] [PMID]
- Philippon MJ, Schenker ML, Briggs KK, Kuppersmith DA, Maxwell RB, Stubbs AJ. Revision hip arthroscopy. Am J Sports Med. 2007; 35(11):1918-21. [DOI:10.1177/0363546507305097] [PMID]
- Zaltz I, Kelly BT, Hetsroni I, Bedi A. The crossover sign overestimates acetabular retroversion. Clin Orthop Relat Res. 2013; 471(8):2463-70. [DOI:10.1007/s11999-012-2689-5] [PMID] [PMCID]
- Zhu X, Yu J, Huang Z. Low-dose chest CT: Optimizing radiation protection for patients. AJR Am J Roentgenol. 2004; 183(3):809-16. [DOI:10.2214/ajr.183.3.1830809] [PMID]
- Sagara Y, Hara AK, Pavlicek W, Silva AC, Paden RG, Wu Q. Abdominal CT: Comparison of low-dose CT with adaptive statistical iterative reconstruction and routine-dose CT with filtered back projection in 53 patients. AJR Am J Roentgenol. 2010; 195(3):713-9. [DOI:10.2214/AJR.09.2989] [PMID]
- Stolzmann P, Donati OF, Scheffel H, Azemaj N, Baumueller S, Plass A, et al. Low-dose CT coronary angiography for the prediction of myocardial ischaemia. Eur Radiol. 2010; 20(1):56-64. [DOI:10.1007/s00330-009-1536-x] [PMID]
- Scheffel H, Alkadhi H, Leschka S, Plass A, Desbiolles L, Guber I, et al. Low-dose CT coronary angiography in the step-and-shoot mode: Diagnostic performance. Heart. 2008; 94(9):1132-7. [DOI:10.1136/hrt.2008.149971] [PMID]
- Dewes P, Frellesen C, Scholtz JE, Fischer S, Vogl TJ, Bauer RW, et al. Low-dose abdominal computed tomography for detection of urinary stone disease-Impact of additional spectral shaping of the X-ray beam on image quality and dose parameters. Eur J Radiol. 2016; 85(6):1058-62. [DOI:10.1016/j.ejrad.2016.03.016] [PMID]
- Su AW, Hillen TJ, Eutsler EP, Bedi A, Ross JR, Larson CM, et al. Low-dose computed tomography reduces radiation exposure by 90% compared with traditional computed tomography among patients undergoing hip-preservation surgery. Arthroscopy. 2019; 35(5):1385-92. [DOI:10.1016/j.arthro.2018.11.013] [PMID] [PMCID]
- Mawatari T, Hayashida Y, Katsuragawa S, Yoshimatsu Y, Hamamura T, Anai K, et al. The effect of deep convolutional neural networks on radiologists' performance in the detection of hip fractures on digital pelvic radiographs. Eur J Radiol. 2020; 130:109188. [DOI:10.1016/j.ejrad.2020.109188] [PMID]
- Yamada Y, Maki S, Kishida S, Nagai H, Arima J, Yamakawa N, et al. Automated classification of hip fractures using deep convolutional neural networks with orthopedic surgeon-level accuracy: Ensemble decision-making with antero-posterior and lateral radiographs. Acta Orthop. 2020; 91(6):699-704. [DOI:10.1080/17453674.2020.1803664] [PMID] [PMCID]
- Murphy EA, Ehrhardt B, Gregson CL, von Arx OA, Hartley A, Whitehouse MR, et al. Machine learning outperforms clinical experts in classification of hip fractures. Sci Rep. 2022; 12(1):2058. [DOI:10.1038/s41598-022-06018-9] [PMID] [PMCID]
- Yu JS, Yu SM, Erdal BS, Demirer M, Gupta V, Bigelow M, et al. Detection and localisation of hip fractures on anteroposterior radiographs with artificial intelligence: Proof of concept. Clin Radiol. 2020; 75(3):237.e1-9. [DOI:10.1016/j.crad.2019.10.022] [PMID]
- Mutasa S, Varada S, Goel A, Wong TT, Rasiej MJ. Advanced deep learning techniques applied to automated femoral neck fracture detection and classification. J Digit Imaging. 2020; 33(5):1209-17. [DOI:10.1007/s10278-020-00364-8] [PMID] [PMCID]
- Beyaz S, Açıcı K, Sümer E. Femoral neck fracture detection in X-ray images using deep learning and genetic algorithm approaches. Jt Dis Relat Surg. 2020; 31(2):175-83. [DOI:10.5606/ehc.2020.72163] [PMID] [PMCID]
- Cheng CT, Chen CC, Cheng FJ, Chen HW, Su YS, Yeh CN, et al. A Human-algorithm integration system for hip fracture detection on plain radiography: System development and validation study. JMIR Med Inform. 2020; 8(11):e19416. [DOI:10.2196/19416] [PMID] [PMCID]
- Bae J, Yu S, Oh J, Kim TH, Chung JH, Byun H, et al. External validation of deep learning algorithm for detecting and visualizing femoral neck fracture including displaced and non-displaced fracture on plain X-ray. J Digit Imaging. 2021; 34(5):1099-09. [DOI:10.1007/s10278-021-00499-2] [PMID] [PMCID]
- Urakawa T, Tanaka Y, Goto S, Matsuzawa H, Watanabe K, Endo N. Detecting intertrochanteric hip fractures with orthopedist-level accuracy using a deep convolutional neural network. Skeletal Radiol. 2019; 48(2):239-244. [DOI:10.1007/s00256-018-3016-3] [PMID]
- Adams M, Chen W, Holcdorf D, McCusker MW, Howe PD, Gaillard F. Computer vs human: Deep learning versus perceptual training for the detection of neck of femur fractures. J Med Imaging Radiat Oncol. 2019; 63(1):27-32. [DOI:10.1111/1754-9485.12828] [PMID]
- Sato Y, Takegami Y, Asamoto T, Ono Y, Hidetoshi T, Goto R, et al. Artificial intelligence improves the accuracy of residents in the diagnosis of hip fractures: A multicenter study. BMC Musculoskelet Disord. 2021; 22(1):407. [DOI:10.1186/s12891-021-04260-2] [PMID] [PMCID]
- Cheng CT, Ho TY, Lee TY, Chang CC, Chou CC, Chen CC, et al. Application of a deep learning algorithm for detection and visualization of hip fractures on plain pelvic radiographs. Eur Radiol. 2019; 29(10):5469-77. [DOI:10.1007/s00330-019-06167-y] [PMID] [PMCID]
- Yoon SJ, Hyong Kim T, Joo SB, Eel Oh S. Automatic multi-class intertrochanteric femur fracture detection from CT images based on AO/OTA classification using faster R-CNN-BO method. J Appl Biomed. 2020; 18(4):97-105. [DOI:10.32725/jab.2020.013] [PMID]
- Maffulli N, Rodriguez HC, Stone IW, Nam A, Song A, Gupta M, et al. Artificial intelligence and machine learning in orthopedic surgery: A systematic review protocol. J Orthop Surg Res. 2020; 15(1):478. [DOI:10.1186/s13018-020-02002-z] [PMID] [PMCID]
- Kakavas G, Malliaropoulos N, Pruna R, Maffulli N. Artificial intelligence: A tool for sports trauma prediction. Injury. 2020; 51(Suppl 3):S63-5. [DOI:10.1016/j.injury.2019.08.033] [PMID]
- Matin P. Basic principles of nuclear medicine techniques for detection and evaluation of trauma and sports medicine injuries. Semin Nucl Med. 1988; 18(2):90-112. [DOI:10.1016/S0001-2998(88)80003-5] [PMID]
- Matin P. The appearance of bone scans following fractures, including immediate and long-term studies. J Nucl Med. 1979; 20(12):1227-31. [Link]
- Bellah RD, Summerville DA, Treves ST, Micheli LJ. Low-back pain in adolescent athletes: Detection of stress injury to the pars interarticularis with SPECT. Radiology. 1991; 180(2):509-12. [DOI:10.1148/radiology.180.2.1829845] [PMID]
- Gholamrezanezhad A, Basques K, Batouli A, Olyaie M, Matcuk G, Alavi A, et al. Non-oncologic applications of PET/CT and PET/MR in musculoskeletal, orthopedic, and rheumatologic imaging: General considerations, techniques, and radiopharmaceuticals. J Nucl Med Technol. 2017; jnmt.117.198663. [DOI:10.2967/jnmt.117.198663] [PMID]
- Gholamrezanezhad A, Basques K, Batouli A, Matcuk G, Alavi A, Jadvar H. Clinical nononcologic Applications of PET/CT and PET/MRI in musculoskeletal, orthopedic, and rheumatologic imaging. AJR Am J Roentgenol. 2018; 210(6):W245-63. [DOI:10.2214/AJR.17.18523] [PMID] [PMCID]
- Andersen KF, Jensen KE, Loft A. PET/MR Imaging in Musculoskeletal Disorders. PET Clin. 2016; 11(4):453-63. [DOI:10.1016/j.cpet.2016.05.007] [PMID]
- Basu S, Kwee TC, Saboury B, Garino JP, Nelson CL, Zhuang H, et al. FDG PET for diagnosing infection in hip and knee prostheses: prospective study in 221 prostheses and subgroup comparison with combined (111)In-labeled leukocyte/(99m)Tc-sulfur colloid bone marrow imaging in 88 prostheses. Clin Nucl Med. 2014; 39(7):609-15. [DOI:10.1097/RLU.0000000000000464] [PMID] [PMCID]
- Vanquickenborne B, Maes A, Nuyts J, Van Acker F, Stuyck J, Mulier M, et al. The value of (18)FDG-PET for the detection of infected hip prosthesis. Eur J Nucl Med Mol Imaging. 2003; 30(5):705-15. [DOI:10.1007/s00259-002-1109-6] [PMID]
- Van Acker F, Nuyts J, Maes A, Vanquickenborne B, Stuyck J, Bellemans J, et al. FDG-PET, 99mtc-HMPAO white blood cell SPET and bone scintigraphy in the evaluation of painful total knee arthroplasties. Eur J Nucl Med. 2001; 28(10):1496-504. [DOI:10.1007/s002590100603] [PMID]
- Shemesh S, Kosashvili Y, Groshar D, Bernstine H, Sidon E, Cohen N, et al. The value of 18-FDG PET/CT in the diagnosis and management of implant-related infections of the tibia: A case series. Injury. 2015; 46(7):1377-82. [DOI:10.1016/j.injury.2015.03.002] [PMID]
- Wang Z, Agrawal P, Zhang YS. Nanotechnologies and nanomaterials in 3D (Bio) printing toward bone regeneration. Adv NanoBiomed Res. 2021; 1(11):2100035. [DOI:10.1002/anbr.202100035]
- Malik S, Muhammad K, Waheed Y. Emerging applications of nanotechnology in healthcare and medicine. Molecules. 2023; 28(18):6624. [DOI:10.3390/molecules28186624] [PMID] [PMCID]
- Pardi N, Tuyishime S, Muramatsu H, Kariko K, Mui BL, Tam YK, et al. Expression kinetics of nucleoside-modified mRNA delivered in lipid nanoparticles to mice by various routes. J Control Release. 2015; 217:345-51. [DOI:10.1016/j.jconrel.2015.08.007] [PMID] [PMCID]
- Hajiali H, Ouyang L, Llopis-Hernandez V, Dobre O, Rose FRAJ. Review of emerging nanotechnology in bone regeneration: Progress, challenges, and perspectives. Nanoscale. 2021; 13(23):10266-80. [DOI:10.1039/D1NR01371H] [PMID]
- Gu W, Wu C, Chen J, Xiao Y. Nanotechnology in the targeted drug delivery for bone diseases and bone regeneration. Int J Nanomedicine. 2013; 8:2305-17. [DOI:10.2147/IJN.S44393] [PMID] [PMCID]
- Wen J, Cai D, Gao W, He R, Li Y, Zhou Y, et al. Osteoimmunomodulatory Nanoparticles for Bone Regeneration. Nanomaterials. 2023; 13(4):692. [DOI:10.3390/nano13040692] [PMID] [PMCID]
Type of Study: Review Paper |
Subject:
Hip surgery
Received: 2022/12/9 | Accepted: 2023/01/7 | Published: 2023/11/1
Received: 2022/12/9 | Accepted: 2023/01/7 | Published: 2023/11/1
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |