Volume 10, Issue 2 (5-2023)
JROS 2023, 10(2): 75-80 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Saffarpour S, Mirzaie A, Raja F, Tehrani-Banihashemi A, Heydari P, Zareie B et al . Serum Inflammatory Markers in Osteoporotic Fracture Patients: A Survey in the Fracture Liaison Service at Shafayahyaeian Orthopedic Hospital, Tehran Province, Iran. JROS 2023; 10 (2) :75-80
URL: http://jros.iums.ac.ir/article-1-2228-en.html
URL: http://jros.iums.ac.ir/article-1-2228-en.html
Sepideh Saffarpour1 

 , Alireza Mirzaie2
, Alireza Mirzaie2 
 , Fatemeh Raja1
, Fatemeh Raja1 
 , Arash Tehrani-Banihashemi3
, Arash Tehrani-Banihashemi3 
 , Pegah Heydari1
, Pegah Heydari1 
 , Bushra Zareie1
, Bushra Zareie1 
 , Mozhdeh Zabihiyeganeh1
, Mozhdeh Zabihiyeganeh1 




 , Alireza Mirzaie2
, Alireza Mirzaie2 
 , Fatemeh Raja1
, Fatemeh Raja1 
 , Arash Tehrani-Banihashemi3
, Arash Tehrani-Banihashemi3 
 , Pegah Heydari1
, Pegah Heydari1 
 , Bushra Zareie1
, Bushra Zareie1 
 , Mozhdeh Zabihiyeganeh1
, Mozhdeh Zabihiyeganeh1 


1- Department of Orthopedics, Bone and Joint Reconstruction Research Center, School of Medicine, Iran University of Medical Sciences, Tehran, Iran.
2- Department of Orthopedic Surgery, University of Minnesota, Minneapolis, United States.
3- Department of Community and Family Medicine, School of Medicine, Iran University of Medical Sciences, Tehran, Iran.
2- Department of Orthopedic Surgery, University of Minnesota, Minneapolis, United States.
3- Department of Community and Family Medicine, School of Medicine, Iran University of Medical Sciences, Tehran, Iran.
Full-Text [PDF 509 kb]
(154 Downloads)
| Abstract (HTML) (855 Views)
Full-Text: (163 Views)
Introduction
Osteoporosis is a significant health issue among the elderly and a leading cause of morbidity and mortality. A fracture liaison service (FLS) is a specialized care model in which a coordinator identifies patients with fractures and assesses their fracture risk to facilitate effective osteoporosis treatment for high-risk individuals [1]. The primary objective of FLS is to prevent secondary fractures by ensuring that patients with fractures receive the necessary osteoporosis care to prevent refractures. The key objectives of FLS include identification, investigation, and initiation of appropriate treatment. The FLS program was implemented at Shafayahyayan Hospital in October 2020 to enhance osteoporosis care and fracture clinical outcomes.
According to literature, secondary causes of osteoporosis can affect two-thirds of older men and postmenopausal women. Secondary causes of bone loss can involve various underlying processes and medical conditions and the use of certain medications that can impact the achievement of peak bone mass during young adulthood or lead to excessive bone resorption, affecting bone health and quality. During the evaluation of secondary causes, in addition to a comprehensive medical history and bone mineral density tests, laboratory tests related to the causes of secondary osteoporosis are required. This is because serious diseases, such as multiple myeloma and monoclonal gammopathy of uncertain significance, can go undiagnosed, and osteoporotic fractures may occur in individuals with these conditions. However, an increase in inflammatory markers can be caused by inflammatory and infectious factors, such as coronavirus infection. The inflammatory process, a physiological factor, should also be considered [2-4].
Inflammatory markers, such as C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) are used to screen for probable monoclonal gammopathy or other underlying inflammatory diseases. CRP and ESR are standard hematology tests that may indicate increased inflammatory activity in the body caused by one or more conditions, such as autoimmune disease, infection, or malignancy. These tests are not specific diagnostic tools for a particular illness but are performed in combination with other tests to determine the presence of increased inflammatory activity [5, 6].
This study assessed ESR and CRP as part of a secondary osteoporosis workup to rule out secondary osteoporosis causes in patients with fractures, including autoimmune disease, infection, or malignancy and their associations with patients’ demographic and clinical characteristics.
Methods
The medical records of patients referred to the FLS clinic of the Shafayahyayan Hospital from October 2020 to May 2023 with osteoporotic fractures were retrospectively reviewed after obtaining the necessary permission from the Ethics Committee of Iran University of Medical Sciences. Before enrolling in the FLS, patients signed an informed consent form allowing anonymous use of their medical data for publication. The inclusion criteria included age >50 years and non-traumatic fracture. The exclusion criteria included patients with specific infections, cancer, or rheumatic diseases. First, the demographic and clinical characteristics of the patients, including sex, age, bone mass density (BMD T-score), body mass index (BMI), glucocorticoid medication use, fracture location, and serum level of vitamin D, were extracted from their medical records and the relationship between them and high ESR and CRP was evaluated.
ESR was determined using the Westergren method. Briefly, 200 mL of the patient’s blood was transferred to a Westergren-Katz tube and maintained vertically at room temperature for approximately an hour. After this period, the distance between the sedimented erythrocytes and measured supernatant plasma was reported as the ESR value. Considering that the ESR level is related to age >30 mm/h is considered a high ESR [7].
We added one drop of CRP latex reagent to the patient’s serum sample and mixed it with a wooden applicator to measure the CRP level. After 2 min, agglutination was observed, indicating the presence of CRP in the serum. A concentration of >10 mg/L was considered high [8].
Statistical analysis
Data on qualitative variables were established using frequency and percentage indicators, whereas quantitative variables were specified using Mean±SD indicators. The normality of the quantitative data distribution was measured using the Kolmogorov–Smirnov test, and parametric or non-parametric proportional tests were applied to evaluate the relationship between variables depending on their type and distribution. Statistical analysis was performed using the SPSS software version 26, and the significance level was SET at P<0.05.
Results
Table 1 presents the demographic characteristics of 1979 patients with previous osteoporotic fragility fractures selected for the study at the Shafa-FLS clinic.
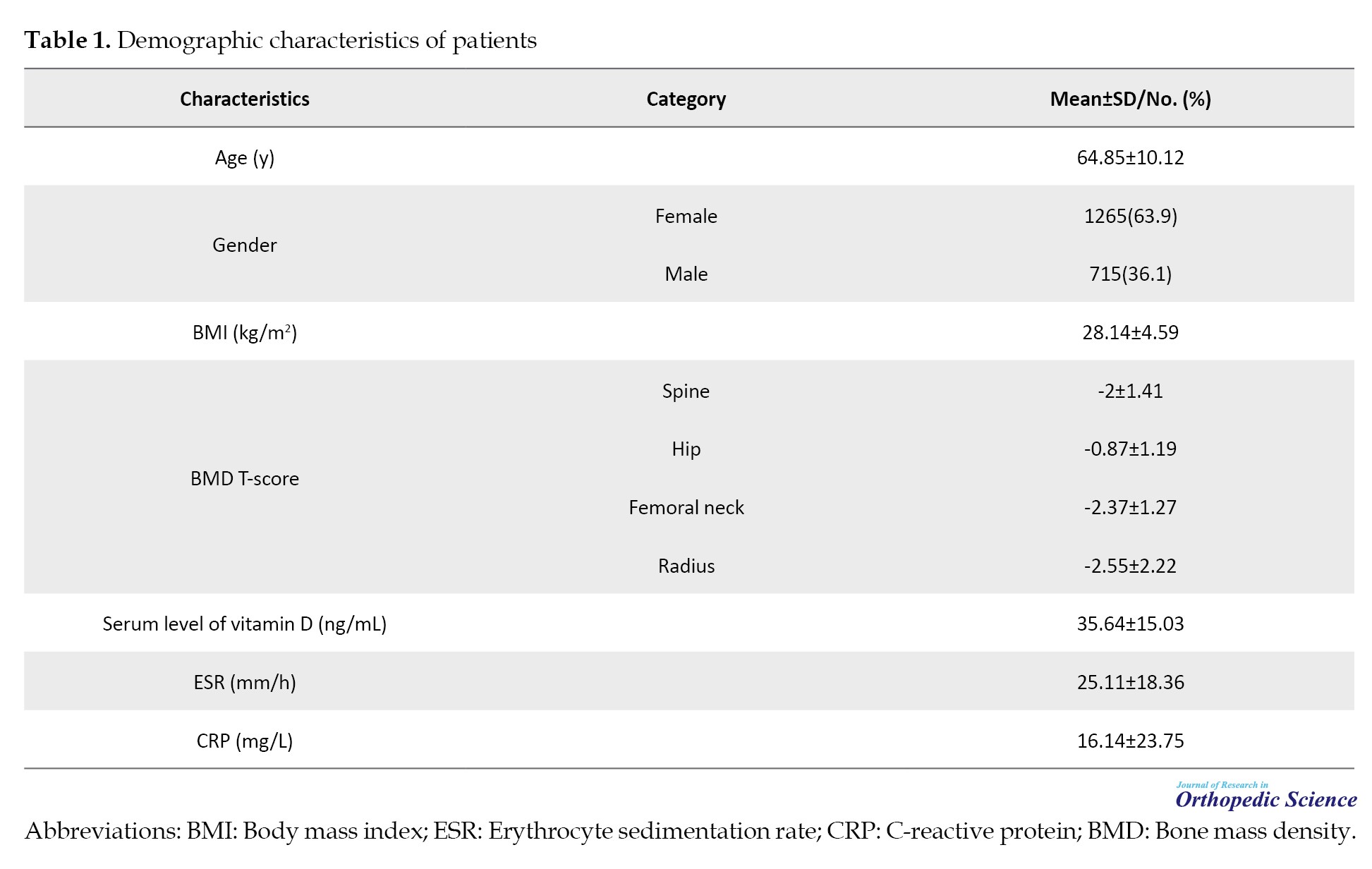
Based on the American Association of Clinical Endocrinology (AACE) osteoporosis guideline definition, 62% of the study population was classified as osteoporosis, 33% as osteopenia, and only 5% as usual across three regions of BMD [3].
The ESR level was significant between men and women (P<0.001). The average ESR in women was 28.43±18.14 mm/h, while that in men was 19.01±17.23 mm/h. The highest ESR recorded was 102 mm/h. Approximately 32% of patients had high ESR levels (>30 mm/h). The frequency distribution of high ESR in different states of bone density was as follows: Osteoporotic, 30%; osteopenic, 22%; regular, 23%. Although high ESR was more prevalent in the osteoporotic group, the difference was insignificant (P>0.05).
The data showed that the mean CRP level was 16±23 mg/L, with a maximum value of 130 mg/L. Using a cutoff of 10 mg/L for elevated CRP, 40% of patients had a high value. Men had a significantly higher mean CRP (18.36±26.39 mg/L) than women (14.87±22.05 mg/L) (P=0.003).
Table 2 presents the influence of various variables, including sex, age, vitamin levels, BMI, hemoglobin levels, and spine radius bone status, on the incidence of elevated CRP and ESR in patients with fractures.
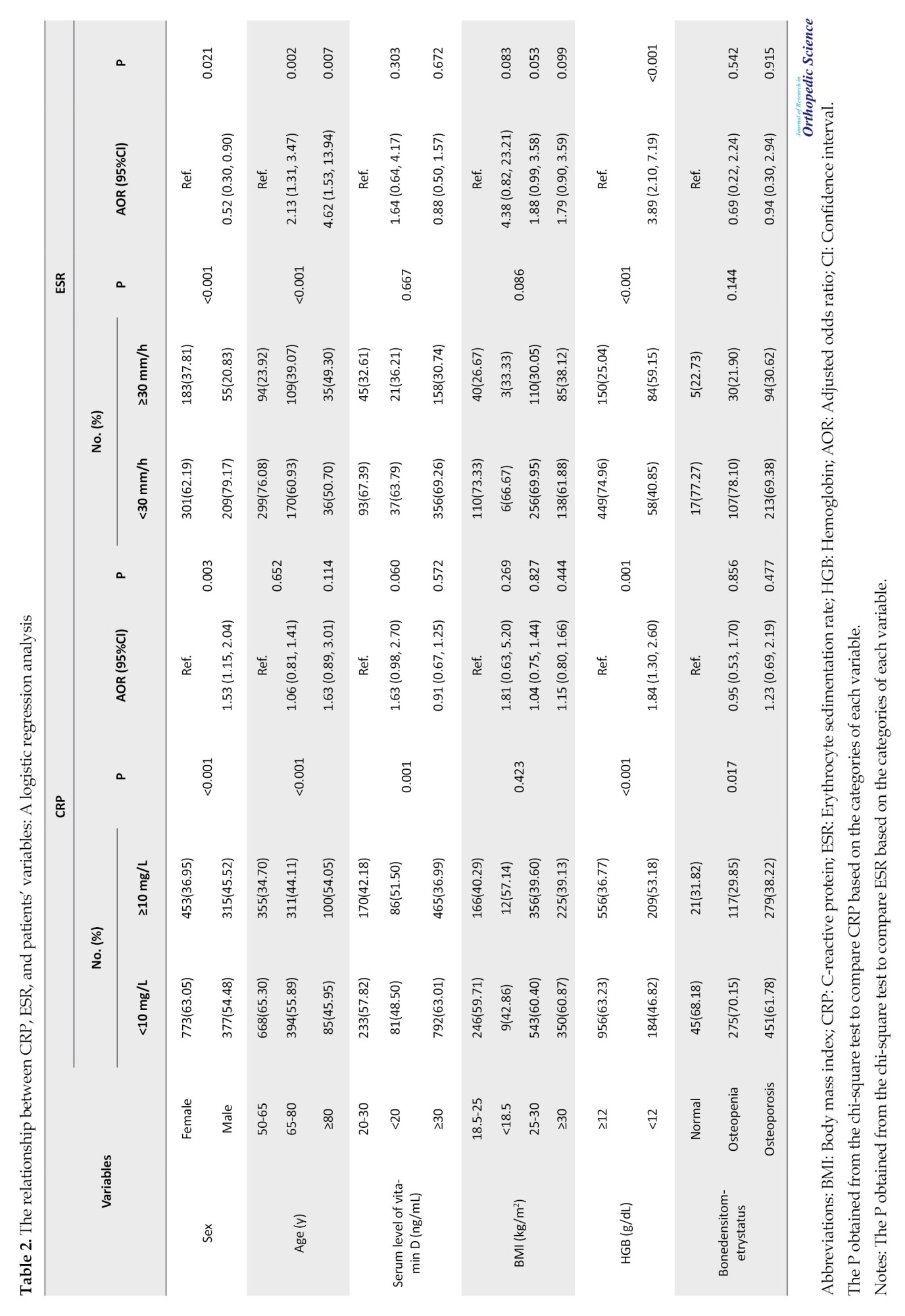
The results indicated that being male and having low hemoglobin levels were significantly associated with increased CRP levels. Conversely, being female, over 64 years, and presenting with low hemoglobin levels were significantly correlated with elevated ESR levels.
Patients with osteoporosis had a significantly higher frequency of elevated CRP than those with osteopenia or normal bone density. Specifically, 38% of osteoporotic patients had high CRP compared to 30% of osteopenic patients and 32% of patients with normal bone density (P=0.017) (Table 2).
Regarding the relationship between CRP and other variables, the study found a statistically significant relationship between CRP levels and age (r=0.180, P<0.001), BMI (r=-0.059, P=0.010), and bone mineral density in the radius area (r=0.065, P=0.022). Furthermore, a significant relationship was observed between serum vitamin D level and CRP (r=-0.059, P=0.011). However, the correlation coefficient between these variables and CRP was negligible.
No cases of secondary osteoporosis were found in patients with high inflammatory markers during their 2-year follow-up.
Discussion
Considering the high prevalence of secondary osteoporosis and its crucial causes, it is necessary to investigate and diagnose this condition [2-4]. In this study, in addition to the surgical treatment of acute fractures, the secondary causes of osteoporosis were investigated in men over 50 years of age and postmenopausal women referred to Shafayahyayan Hospital due to fractures caused by osteoporosis. In this study, ESR and CRP were measured as inflammatory markers due to the spread of the coronavirus in 2020 and 2021; patients with high inflammatory markers were at a higher risk of virus transmission. Additionally, monoclonal gammopathy and fractures due to cancer are also concern for these patients. The results showed that 32% of patients had elevated ESR and 40% had elevated CRP levels. Fractured patients had even higher levels, with some showing ESR levels over 100 mm/h and CRP levels up to 130. No cases of secondary osteoporosis were reported during the two-year follow-up of patients with Shafa-FLS.
The influence of BMI on fracture risk is still being determined owing conflicting data. Low BMI has often been identified as a risk factor for fragility fractures due to increased fall risk owing to muscle weakness and decreased soft tissue that defends bones from impact forces. Recent studies have indicated a possible relationship between higher BMI and increased risk of fracture, particularly in non-hip locations [9-11]. However, another study reported no direct correlation between BMI and fracture risk. The impact of BMI on fracture risk is primarily determined by femoral neck bone density in both sexes. In the absence of BMD, the contribution of BMI to fracture prediction is minimal [12].
The mean age of the study population was 65 years. Hence, inflammation and age-related fragility should be considered when analyzing the results [13]. This study demonstrated a negligible correlation between age and inflammatory markers.
On the other hand, inflammation is a critical response in bone healing. An animal model demonstrated impaired bone healing after increased pro-inflammatory markers. Another study found that increased anti-inflammatory IL-10 improves osseous healing post-fractures in rats. In humans, systemic inflammatory conditions, such as arthritis, diabetes mellitus, sepsis, or multiple traumas, can impair osseous healing. While excessive inflammation worsens healing, impaired inflammation can impede healing and increase rates of delayed osseous healing [4, 14, 15].
Inflammation is a natural part of the healing process in the first week after a fracture. Since the examination of inflammatory markers in our patients occurred in the first few days after the fracture, the high levels of these markers can be a normal fracture repair reaction [4, 16].
Intravenous bisphosphonates are the preferred initial treatment for elderly patients with fractures. However, these medications can cause side effects, such as fever, bone pain, and a severe inflammatory response that may activate gamma-delta T lymphocytes and lead to the development of inflammatory symptoms after zoledronic acid injection [17, 18]. Therefore, it is reasonable to delay the administration of zoledronic acid until the inflammatory markers decrease, typically after the first week in cases of acute fracture.
Conclusion
About one-third of patients had elevated inflammatory markers; however after a two-year follow-up, none showed any symptoms of underlying diseases, including monoclonal gammopathy. Elevated levels of inflammatory markers in the initial days following the fracture result from the physiological process of fracture repair. However, considering the advanced age of patients, inflammation should also be considered a potential cause of increased inflammatory markers.
The current study has some limitations. To observe the decreasing trend in these tests, it would have been ideal to repeat the inflammatory markers within the next few weeks. However, this is not feasible.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of Iran University of Medical Sciences, Tehran, Iran (Code: IR.IUMS.REC.1401.591). Informed consent was obtained from all participants.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Data collection: Pegah Heydari; Statistical analysis: Alireza Mirzaei, and Bushra Zareie; Writing the original draft: Sepideh Saffarpour; Study design, review and editing: Mozhdeh Zabihiyeganeh.
Conflict of interest
The authors declared no conflict of interest.
References
Osteoporosis is a significant health issue among the elderly and a leading cause of morbidity and mortality. A fracture liaison service (FLS) is a specialized care model in which a coordinator identifies patients with fractures and assesses their fracture risk to facilitate effective osteoporosis treatment for high-risk individuals [1]. The primary objective of FLS is to prevent secondary fractures by ensuring that patients with fractures receive the necessary osteoporosis care to prevent refractures. The key objectives of FLS include identification, investigation, and initiation of appropriate treatment. The FLS program was implemented at Shafayahyayan Hospital in October 2020 to enhance osteoporosis care and fracture clinical outcomes.
According to literature, secondary causes of osteoporosis can affect two-thirds of older men and postmenopausal women. Secondary causes of bone loss can involve various underlying processes and medical conditions and the use of certain medications that can impact the achievement of peak bone mass during young adulthood or lead to excessive bone resorption, affecting bone health and quality. During the evaluation of secondary causes, in addition to a comprehensive medical history and bone mineral density tests, laboratory tests related to the causes of secondary osteoporosis are required. This is because serious diseases, such as multiple myeloma and monoclonal gammopathy of uncertain significance, can go undiagnosed, and osteoporotic fractures may occur in individuals with these conditions. However, an increase in inflammatory markers can be caused by inflammatory and infectious factors, such as coronavirus infection. The inflammatory process, a physiological factor, should also be considered [2-4].
Inflammatory markers, such as C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) are used to screen for probable monoclonal gammopathy or other underlying inflammatory diseases. CRP and ESR are standard hematology tests that may indicate increased inflammatory activity in the body caused by one or more conditions, such as autoimmune disease, infection, or malignancy. These tests are not specific diagnostic tools for a particular illness but are performed in combination with other tests to determine the presence of increased inflammatory activity [5, 6].
This study assessed ESR and CRP as part of a secondary osteoporosis workup to rule out secondary osteoporosis causes in patients with fractures, including autoimmune disease, infection, or malignancy and their associations with patients’ demographic and clinical characteristics.
Methods
The medical records of patients referred to the FLS clinic of the Shafayahyayan Hospital from October 2020 to May 2023 with osteoporotic fractures were retrospectively reviewed after obtaining the necessary permission from the Ethics Committee of Iran University of Medical Sciences. Before enrolling in the FLS, patients signed an informed consent form allowing anonymous use of their medical data for publication. The inclusion criteria included age >50 years and non-traumatic fracture. The exclusion criteria included patients with specific infections, cancer, or rheumatic diseases. First, the demographic and clinical characteristics of the patients, including sex, age, bone mass density (BMD T-score), body mass index (BMI), glucocorticoid medication use, fracture location, and serum level of vitamin D, were extracted from their medical records and the relationship between them and high ESR and CRP was evaluated.
ESR was determined using the Westergren method. Briefly, 200 mL of the patient’s blood was transferred to a Westergren-Katz tube and maintained vertically at room temperature for approximately an hour. After this period, the distance between the sedimented erythrocytes and measured supernatant plasma was reported as the ESR value. Considering that the ESR level is related to age >30 mm/h is considered a high ESR [7].
We added one drop of CRP latex reagent to the patient’s serum sample and mixed it with a wooden applicator to measure the CRP level. After 2 min, agglutination was observed, indicating the presence of CRP in the serum. A concentration of >10 mg/L was considered high [8].
Statistical analysis
Data on qualitative variables were established using frequency and percentage indicators, whereas quantitative variables were specified using Mean±SD indicators. The normality of the quantitative data distribution was measured using the Kolmogorov–Smirnov test, and parametric or non-parametric proportional tests were applied to evaluate the relationship between variables depending on their type and distribution. Statistical analysis was performed using the SPSS software version 26, and the significance level was SET at P<0.05.
Results
Table 1 presents the demographic characteristics of 1979 patients with previous osteoporotic fragility fractures selected for the study at the Shafa-FLS clinic.

Based on the American Association of Clinical Endocrinology (AACE) osteoporosis guideline definition, 62% of the study population was classified as osteoporosis, 33% as osteopenia, and only 5% as usual across three regions of BMD [3].
The ESR level was significant between men and women (P<0.001). The average ESR in women was 28.43±18.14 mm/h, while that in men was 19.01±17.23 mm/h. The highest ESR recorded was 102 mm/h. Approximately 32% of patients had high ESR levels (>30 mm/h). The frequency distribution of high ESR in different states of bone density was as follows: Osteoporotic, 30%; osteopenic, 22%; regular, 23%. Although high ESR was more prevalent in the osteoporotic group, the difference was insignificant (P>0.05).
The data showed that the mean CRP level was 16±23 mg/L, with a maximum value of 130 mg/L. Using a cutoff of 10 mg/L for elevated CRP, 40% of patients had a high value. Men had a significantly higher mean CRP (18.36±26.39 mg/L) than women (14.87±22.05 mg/L) (P=0.003).
Table 2 presents the influence of various variables, including sex, age, vitamin levels, BMI, hemoglobin levels, and spine radius bone status, on the incidence of elevated CRP and ESR in patients with fractures.

The results indicated that being male and having low hemoglobin levels were significantly associated with increased CRP levels. Conversely, being female, over 64 years, and presenting with low hemoglobin levels were significantly correlated with elevated ESR levels.
Patients with osteoporosis had a significantly higher frequency of elevated CRP than those with osteopenia or normal bone density. Specifically, 38% of osteoporotic patients had high CRP compared to 30% of osteopenic patients and 32% of patients with normal bone density (P=0.017) (Table 2).
Regarding the relationship between CRP and other variables, the study found a statistically significant relationship between CRP levels and age (r=0.180, P<0.001), BMI (r=-0.059, P=0.010), and bone mineral density in the radius area (r=0.065, P=0.022). Furthermore, a significant relationship was observed between serum vitamin D level and CRP (r=-0.059, P=0.011). However, the correlation coefficient between these variables and CRP was negligible.
No cases of secondary osteoporosis were found in patients with high inflammatory markers during their 2-year follow-up.
Discussion
Considering the high prevalence of secondary osteoporosis and its crucial causes, it is necessary to investigate and diagnose this condition [2-4]. In this study, in addition to the surgical treatment of acute fractures, the secondary causes of osteoporosis were investigated in men over 50 years of age and postmenopausal women referred to Shafayahyayan Hospital due to fractures caused by osteoporosis. In this study, ESR and CRP were measured as inflammatory markers due to the spread of the coronavirus in 2020 and 2021; patients with high inflammatory markers were at a higher risk of virus transmission. Additionally, monoclonal gammopathy and fractures due to cancer are also concern for these patients. The results showed that 32% of patients had elevated ESR and 40% had elevated CRP levels. Fractured patients had even higher levels, with some showing ESR levels over 100 mm/h and CRP levels up to 130. No cases of secondary osteoporosis were reported during the two-year follow-up of patients with Shafa-FLS.
The influence of BMI on fracture risk is still being determined owing conflicting data. Low BMI has often been identified as a risk factor for fragility fractures due to increased fall risk owing to muscle weakness and decreased soft tissue that defends bones from impact forces. Recent studies have indicated a possible relationship between higher BMI and increased risk of fracture, particularly in non-hip locations [9-11]. However, another study reported no direct correlation between BMI and fracture risk. The impact of BMI on fracture risk is primarily determined by femoral neck bone density in both sexes. In the absence of BMD, the contribution of BMI to fracture prediction is minimal [12].
The mean age of the study population was 65 years. Hence, inflammation and age-related fragility should be considered when analyzing the results [13]. This study demonstrated a negligible correlation between age and inflammatory markers.
On the other hand, inflammation is a critical response in bone healing. An animal model demonstrated impaired bone healing after increased pro-inflammatory markers. Another study found that increased anti-inflammatory IL-10 improves osseous healing post-fractures in rats. In humans, systemic inflammatory conditions, such as arthritis, diabetes mellitus, sepsis, or multiple traumas, can impair osseous healing. While excessive inflammation worsens healing, impaired inflammation can impede healing and increase rates of delayed osseous healing [4, 14, 15].
Inflammation is a natural part of the healing process in the first week after a fracture. Since the examination of inflammatory markers in our patients occurred in the first few days after the fracture, the high levels of these markers can be a normal fracture repair reaction [4, 16].
Intravenous bisphosphonates are the preferred initial treatment for elderly patients with fractures. However, these medications can cause side effects, such as fever, bone pain, and a severe inflammatory response that may activate gamma-delta T lymphocytes and lead to the development of inflammatory symptoms after zoledronic acid injection [17, 18]. Therefore, it is reasonable to delay the administration of zoledronic acid until the inflammatory markers decrease, typically after the first week in cases of acute fracture.
Conclusion
About one-third of patients had elevated inflammatory markers; however after a two-year follow-up, none showed any symptoms of underlying diseases, including monoclonal gammopathy. Elevated levels of inflammatory markers in the initial days following the fracture result from the physiological process of fracture repair. However, considering the advanced age of patients, inflammation should also be considered a potential cause of increased inflammatory markers.
The current study has some limitations. To observe the decreasing trend in these tests, it would have been ideal to repeat the inflammatory markers within the next few weeks. However, this is not feasible.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of Iran University of Medical Sciences, Tehran, Iran (Code: IR.IUMS.REC.1401.591). Informed consent was obtained from all participants.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Data collection: Pegah Heydari; Statistical analysis: Alireza Mirzaei, and Bushra Zareie; Writing the original draft: Sepideh Saffarpour; Study design, review and editing: Mozhdeh Zabihiyeganeh.
Conflict of interest
The authors declared no conflict of interest.
References
- Bonanni S, Sorensen AA, Dubin J, Drees B. The role of the fracture liaison service in osteoporosis care. Mo Med. 2017; 114(4):295-98. [PMID]
- Ferrucci L, Fabbri E. Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol. 2018; 15(9):505-22. [DOI:10.1038/s41569-018-0064-2] [PMID] [PMCID]
- Camacho PM, Petak SM, Binkley N, Diab DL, Eldeiry LS, Farooki A, et al. American association of clinical endocrinologists/american college of endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis-2020 update. Endocr Pract. 2020; 26(Suppl 1):1-46. [DOI:10.4158/GL-2020-0524SUPPL] [PMID]
- ElHawary H, Baradaran A, Abi-Rafeh J, Vorstenbosch J, Xu L, Efanov JI. Bone healing and inflammation: principles of fracture and repair. Semin Plast Surg. 2021; 35(3):198-203. [DOI:10.1055/s-0041-1732334] [PMID] [PMCID]
- Lacativa PG, de Farias ML. Office practice of osteoporosis evaluation. Arq Bras Endocrinol Metabol. 2006; 50(4):674-84. [DOI:10.1590/S0004-27302006000400013] [PMID]
- David S, Shoenfeld Y. [Erythrocyte sedimentation rate - purposeful review for clinical application (Hebrew)]. Harefuah. 2022; 161(9):552-55. [PMID]
- Tishkowski K, Gupta V. Erythrocyte sedimentation rate. Treasure Island: StatPearls; 2023. [Link]
- McBride JD, Cooper MA. A high sensitivity assay for the inflammatory marker C-Reactive protein employing acoustic biosensing. J Nanobiotechnology. 2008; 6:5. [DOI:10.1186/1477-3155-6-5] [PMID] [PMCID]
- De Laet C, Kanis JA, Odén A, Johanson H, Johnell O, Delmas P, et al. Body mass index as a predictor of fracture risk: A meta-analysis. Osteoporos Int. 2005; 16(11):1330-8. [DOI:10.1007/s00198-005-1863-y] [PMID]
- Kim J, Lee S, Kim SS, Lee JP, Kim JS, Jung JG, et al. Association between body mass index and fragility fracture in postmenopausal women: A cross-sectional study using Korean National Health and Nutrition Examination Survey 2008-2009 (KNHANES IV). BMC Womens Health. 2021; 21(1):60. [DOI:10.1186/s12905-021-01209-4] [PMID] [PMCID]
- Nunes Cavalcante Castro BA, Torres Dos Reis Neto E, Szejnfeld VL, Szejnfeld J, Marvulle V, de Medeiros Pinheiro M. Could obesity be considered as risk factor for non-vertebral low-impact fractures? Adv Rheumatol. 2018; 58(1):42. [DOI:10.1186/s42358-018-0044-6] [PMID]
- Lv QB, Fu X, Jin HM, Xu HC, Huang ZY, Xu HZ, et al. The relationship between weight change and risk of hip fracture: Meta-analysis of prospective studies. Sci Rep. 2015; 5:16030. [DOI:10.1038/srep16030] [PMID] [PMCID]
- Soysal P, Arik F, Smith L, Jackson SE, Isik AT. Inflammation, frailty and cardiovascular disease. Adv Exp Med Biol. 2020; 1216:55-64. [DOI:10.1007/978-3-030-33330-0_7] [PMID]
- Gai Y, Yin Y, Guan L, Zhang S, Chen J, Yang J, et al. Rational design of bioactive materials for bone hemostasis and defect repair. Cyborg Bionic Syst. 2023; 4:0058. [DOI:10.34133/cbsystems.0058] [PMID] [PMCID]
- Maruyama M, Rhee C, Utsunomiya T, Zhang N, Ueno M, Yao Z, et al. Modulation of the inflammatory response and bone healing. Front Endocrinol. 2020; 11:386. [DOI:10.3389/fendo.2020.00386] [PMID] [PMCID]
- Dittmer KE, Firth EC. Mechanisms of bone response to injury. J Vet Diagn Invest. 2017; 29(4):385-95. [DOI:10.1177/1040638716679861] [PMID]
- Jamil M, Daneshvar A, Nachawati D, El Sharu H, Meysami A. A rare presentation of zoledronate-induced systemic inflammatory response. Cureus. 2023; 15(7):e41524. [DOI:10.7759/cureus.41524]
- Scala R, Maqoud F, Antonacci M, Dibenedetto JR, Perrone MG, Scilimati A, et al. Bisphosphonates targeting ion channels and musculoskeletal effects. Front Pharmacol. 2022; 13:837534. [DOI:10.3389/fphar.2022.837534] [PMID] [PMCID]
Type of Study: Research Article |
Subject:
Rheumatology
Received: 2022/03/3 | Accepted: 2022/09/14 | Published: 2023/05/1
Received: 2022/03/3 | Accepted: 2022/09/14 | Published: 2023/05/1
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |






