Volume 10, Issue 2 (5-2023)
JROS 2023, 10(2): 53-66 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Nakhaei Amroodi M, Bahaeddini M, Amiri S, Karimi M, Mokhtari K, Tabrizian P. Insights Into Gene Therapy Innovations for Orthopedic Disorders: A Mini-review. JROS 2023; 10 (2) :53-66
URL: http://jros.iums.ac.ir/article-1-2241-en.html
URL: http://jros.iums.ac.ir/article-1-2241-en.html
Morteza Nakhaei Amroodi1 

 , Mohammadreza Bahaeddini1
, Mohammadreza Bahaeddini1 

 , Saeedreza Amiri1
, Saeedreza Amiri1 
 , Mansour Karimi1
, Mansour Karimi1 
 , Khatere Mokhtari2
, Khatere Mokhtari2 

 , Pouria Tabrizian1
, Pouria Tabrizian1 




 , Mohammadreza Bahaeddini1
, Mohammadreza Bahaeddini1 

 , Saeedreza Amiri1
, Saeedreza Amiri1 
 , Mansour Karimi1
, Mansour Karimi1 
 , Khatere Mokhtari2
, Khatere Mokhtari2 

 , Pouria Tabrizian1
, Pouria Tabrizian1 


1- Department of Orthopedics, Bone and Joint Reconstruction Research Center, School of Medicine, Shafayahyaeian Hospital, Iran University of Medical Sciences, Tehran, Iran.
2- Department of Cellular and Molecular Biology and Microbiology, Faculty of Biological Science and Technology, University of Isfahan, Isfahan, Iran.
2- Department of Cellular and Molecular Biology and Microbiology, Faculty of Biological Science and Technology, University of Isfahan, Isfahan, Iran.
Keywords: Gene therapy, Orthopedics, Spinal fusion failure, Bone tumors, Genetic disorders, Muscular disorders
Full-Text [PDF 820 kb]
(70 Downloads)
| Abstract (HTML) (478 Views)
Full-Text: (59 Views)
Introduction
Genes act as fundamental building blocks of heredity, governing individuals’ physical traits and functional characteristics. At birth, individuals inherit a predetermined genetic blueprint, which, when coupled with environmental influences, dictates susceptibility to various disease conditions. Gene therapy has emerged as a strategy for correcting aberrant genes associated with disease development, involving the transfer and activation of genes within individuals for therapeutic purposes [1, 2]. Gene therapy consists of the delivery of genes to individuals with a therapeutic aim. Human gene transfer can be achieved through ex vivo and in vivo approaches. Ex vivo gene therapy involves the transfer of genes into cells outside the body, typically in a tissue culture environment. Subsequently, genetically modified cells are reintroduced into the host organism. In contrast, the in vivo approach entails the direct transfer of genes into specific somatic cells of the host organism in its native environment [3]. Ex vivo techniques are notably more intricate but offer relatively higher safety profiles. Additionally, the ex vivo approach provides an opportunity to select cells expressing the desired genes at elevated levels in vitro. In contrast, in vivo gene delivery techniques are more straightforward from a technical standpoint and are currently more widely employed. The application of gene therapy to germline cells, initially envisioned as a remedy for hereditary genetic conditions, raises ethical dilemmas owing to the far-reaching implications of modifications, impacting all future generations and potentially evoking concerns related to eugenics. As a result, while experimental somatic cell gene therapy represents a relatively emerging domain, substantial advancements have been made in the last decade. The widespread recognition of the potential of gene therapy to transform medical practices in the 21st century is evident [4]. This review underscores this innovative approach’s utilization and prospective benefits in tackling orthopedic disorders.
Strategies for Gene Therapy
Gene therapy can be categorized into two main branches: Somatic cells and germline cells. It is crucial to highlight that all clinical trials conducted thus far exclusively examined somatic cell gene therapy [5, 6]. In somatic gene therapy, a plethora of tissues are available for consideration. Additionally, once the target tissue is identified, selecting the target cell in that tissue presents a considerable challenge, often surpassing the complexity of choosing the target tissue itself. The decision regarding which cell to target frequently depends on the method employed for vector delivery [7]. In gene therapy, the synthesis of a functional protein involves several sequential processes. First, exogenous complementary DNA (cDNA) must traverse the cell membrane, evade degradation within the lysosomes, and subsequently access the nucleus for transcription. After transcription, the resulting messenger ribonucleic acid (mRNA) is translated into amino acids, culminating in the production of the intended peptide or protein [8]. Gene therapy holds promise for delivering growth factors in a biologically active manner because the protein is synthesized endogenously in the body. This can result in more accurate post-translational modifications and the formation of tertiary structures with ligands that are more readily recognizable, thereby augmenting their ability to bind to cell surface receptors compared with recombinant proteins [9, 10].
Gene Delivery Vectors
For gene expression to occur, the transferred DNA must successfully enter the host cell’s nucleus, where it can integrate into the host cell’s chromosomes or remain in episomes. Introduction is the process of gene transfer using a viral vector. Viral vectors employed in human clinical trials include retroviruses, adenoviruses, adeno-associated viruses, lentiviruses, and HSVs [11, 12]. The Moloney murine leukemia retrovirus is one of the most well-established viral vectors used in gene therapy. The essential attributes of any viral vector pertinent to gene therapy include simplicity, cost-effectiveness of production, potential for evoking immune responses, integration into the host genome, titer, and safety profile [13]. The specific advantages and limitations of each available viral vector system have been comprehensively examined in the literature [14].
Viral Vectors
Vectors derived from retroviruses
Retroviruses are RNA viruses that replicate via an intermediate step involving DNA [3]. The primary advantage of retroviral vectors is their exceptional efficiency in transferring genes into actively dividing cells. This level of accuracy and durability of gene transfer is unmatched by other types of viruses [15]. Retroviral vectors have a substantial cloning capacity, accommodating up to 8 kilobases (kb) of genetic material, and can be manufactured in significant quantities with relative ease for clinical applications. However, their utility is constrained by factors such as a limited host range, inadequate transduction of non-dividing cells, and potential risk of insertional events that trigger tumorigenesis [16]. These delivery systems can transduce osteoblasts, bone marrow stromal cells, and muscle-derived stem cells, positioning them as promising candidates for skeletal gene therapy [17-19]. Investigators found that periosteal mesenchymal stem cells, engineered to express bone morphogenetic protein (BMP)-7 via retroviral vector manipulation, markedly improve the healing process of critical-sized defects. Researchers in a mouse calvarial model noted synergistic advantages in bone regeneration by co-implanting muscle-derived stem cells, genetically modified ex vivo using retroviral vectors to produce BMP-2/4 and vascular endothelial growth factor (VEGF) [20, 21].
Vectors derived from adenovirus
Adenoviruses, DNA viruses featuring a double-stranded genome approximately 35 kb in length, have been extensively evaluated in both preclinical and clinical trials for gene therapy applications [22]. These vectors can accommodate approximately eight kb of genetic material within an expression cassette. However, newer “gutless” adenoviral vectors can accommodate significantly larger DNA sequences [23]. Adenovirus vectors exhibit remarkable efficiency, facilitating their production in significant quantities and resulting in elevated levels of expression following transduction. Furthermore, they demonstrate the capacity to transfer genes to replicating and non-replicating cells [3]. The primary hurdle associated with using adenoviruses in gene therapy pertains to the immune response elicited by the host, which restricts transgene expression in animals with intact immune systems [10]. Adenoviral vectors expressing lacZ and transforming growth factor β1 (TGF-β1) efficiently transduced osteoblasts and osteoclasts, resulting in notable alterations within the epiphyseal plate [24, 25].
Vectors derived from herpes simplex virus (HSV)
HSV vector, known for its remarkable DNA virus and infectivity, remains latent in nerve cells. However, its extensive genome and cytotoxic properties make it less favorable for consideration as a vector [26]. Researchers have recently employed a second-generation, low-toxicity HSV vector to deliver an interleukin-1 receptor antagonist (IL-1Ra) gene and a soluble tumor necrosis factor-α (TNF-α) receptor gene. This approach significantly reduced arthritis symptoms, primarily through IL-1Ra [27].
Vectors derived from lentiviral
For the most part, lentiviral vectors are derived from human immunodeficiency virus (HIV). Consequently, prioritizing safety is crucial when employing this gene delivery platform. Lentiviral vectors engineered from HIV demonstrate efficient transduction of human macrophages and primary tissues, such as the brain and muscle [28, 29]. Despite persistent safety concerns, the capacity of lentiviral vectors to infect non-dividing osteogenic cells, their compatibility with osteoblast-specific promoters, and their reduced propensity to induce gene silencing or activate host cell genes strongly indicate that these vectors exert a significant influence on future skeletal gene therapy strategies.
Vectors derived from adeno-associated virus
Recently, recombinant adeno-associated viral (AAV) vectors have emerged as promising substitutes for adenoviral and retroviral vectors for gene therapy. AAV vectors demonstrate non-cytotoxic properties, exceptional safety profiles, and the ability to deliver genes to non-dividing cells. They can be integrated explicitly into the 19th chromosome, ensuring targeted gene insertion. Furthermore, they enable prolonged and consistent gene expression and can be produced at high titers, facilitating their application using in vivo methods [1]. The researchers utilized AAV vectors to deliver marker genes to the arthritic knees of mice overexpressing TNF-α using in vivo techniques. AAV has demonstrated effectiveness as a vector and is expected to be applied in a broader spectrum of clinical treatments [30].
Vectors derived from non-viral
There has been a push for developing non-viral delivery systems owing to concerns regarding safety, immunogenicity, and production limitations associated with viral vectors. These systems involve a combination of genes (DNA) and various chemical formulations. The technique referred to as transfection illustrates a form of gene transfer that does not include viruses. Non-viral delivery systems encompass a diverse range of materials, such as plasmids, peptides, positively charged liposomes, DNA complexes with ligands targeting particular cell receptors to enhance cellular uptake, and Gene-gun technology, which employs gold-coated particles loaded with DNA and introducing them into cells through high-speed bombardment [31-33]. However, their effectiveness typically falls short of that of viral vectors. Non-viral delivery systems demonstrate lower efficiency than viral techniques because no intrinsic biological mechanism is found to integrate the desired DNA material into the genome. These approaches can be classified as physical, mechanical, or chemical approaches. The researchers injected plasmid DNA encoding TGF-α1 directly into the muscles of mice with streptococcal cell wall-induced arthritis. They observed substantial suppression of chronic diseases, which was marked by reduced inflammation at the peak of the acute phase. Additionally, cartilage, bone damage, and pannus formation decrease is observed during the chronic phase [34].
Clinical Applications
Cartilage repair
Impairment of adult articular cartilage frequently results in early-onset arthritis, mainly due to the tissue’s limited regenerative ability. This limitation can be attributed to insufficient availability of stem cells, inadequate vascularization, and low cellular turnover [35]. Numerous methods have been devised to enhance the healing of articular cartilage [36, 37]. Mason et al. used a retroviral vector to genetically modify mesenchymal stem cells by introducing a BMP-7 gene. These modified cells were transplanted onto a polyglycolic acid scaffold to a rabbit osteochondral defect [38, 39]. They demonstrated significantly improved healing of the articular defect compared to control groups at both 8 and 12 weeks post-implantation. More recently, researchers have investigated the effects of in vitro gene transfer of insulin-like growth factor-1 (IGF-1), BMP-2, and TGF-β to rabbit articular chondrocytes [39]. Studies indicate that BMP-7 also stimulates the chondrogenic differentiation of precursor cells derived from the periosteum. Research has shown that incorporating periosteal cells genetically modified to express BMP-7 or sonic hedgehog cDNAs improves the healing of osteochondral defects in rabbits [40]. Given their limited intrinsic capacity for cartilage repair and remodeling, gene therapy cells have often been integrated with diverse scaffolds to replicate the architecture of cartilage tissues. This approach has shown promising results in cartilage repair in rabbits, mainly using periosteal mesenchymal stem cells transfected with BMP-7 and sonic hedgehog genes [41].
Meniscus
Various methods, such as sutures, arrows, and staples, have been devised to conserve the menisci. Nonetheless, tears located solely in the vascularized outer third of the meniscus exhibit healing potential [42]. Preconditioning meniscus allografts using viral vectors expressing growth factors holds promise for expediting graft healing and restructuring while mitigating immunogenic responses. The rationale for employing gene-based approaches to preserve and repair the articular cartilage can be extended to the meniscus. Meniscal cells are responsive to adenoviral and retroviral transduction. Specifically, the delivery of TGF-β1 cDNA into these cells in monolayer culture resulted in a significant increase in proteoglycan and collagen production, with no changes detected in the cells’ collagen phenotype [43-47].
Osteoporosis
Osteoporosis leads to reduced bone density and osteopenia [48]. Osteoporosis manifests as two distinct types. Type 1 osteoporosis is characterized by escalated osteoclastogenesis stemming from estrogen depletion, whereas type 2 is characterized by diminished osteogenesis originating from aging marrow stem cells. Gene therapy for type 2 osteoporosis can be implemented using ex vivo methods involving the transduction of marrow stem cells from osteoporotic donors with adenoviral vectors encoding the BMP-2 gene. This potent growth factor promotes bone formation. Studies have shown that genetically engineered cells significantly enhance osteogenic activity in vivo [49]. Systemic intravenous delivery of adenoviral or AAV vectors containing osteoprotegerin (OPG) cDNA results in elevated levels of OPG in circulation, thereby eliciting a sustained anti-osteoporotic effect in mice [50, 51].
Osteopetrosis
Osteopetrosis, a genetic disorder characterized by excessive bone formation and bone marrow obliteration, presents an opposite phenotype to osteoporosis. Surplus bone formation in osteopetrosis is caused by decreased osteoclastogenesis, which is associated with genetic abnormalities affecting the colony-stimulating factor 1 (CSF-1) gene. Gene therapy offers potential by introducing marrow stem cells modified to overexpress the CSF-1 gene, thereby stimulating heightened osteoclastogenesis [52-54].
Spinal fusion
Spinal fusion is a common procedure in spinal surgery, frequently requiring internal fixation devices for temporary stabilization. However, achieving enduring stability requires a successful bone fusion. Nonetheless, the failure rate to achieve robust bone fusion can reach 45%. Although autogenous bone grafts are efficacious, they are constrained in volume and can induce considerable morbidity at the donor site. Research indicated that the morbidity rate associated with harvesting autogenous iliac crest bone grafts may reach 30%. Allograft bone carries risks of antigenicity and disease transmission. Additionally, alloplastic materials are associated with higher infection rate and extrusion and inferior biomechanical properties [55]. Extensive animal studies have demonstrated the exceptional effectiveness of BMPs in enhancing bone formation at fusion sites, offering strong support for advancing BMP gene therapy to clinical applications. Unlike systemic gene therapy, local gene therapy for spinal fusion is less complex because it requires sustained gene expression for only a brief period, potentially less than a week, to trigger the endochondral osteoinduction cascade. Although several hurdles must be overcome before this innovative approach can be clinically deployed, local gene therapy shows promise as a more favorable method of osteoinduction than the administration of pharmacological doses of recombinant or extracted osteoinductive proteins in spinal surgery. With further refinement, BMP gene therapy stands poised to facilitate bony :union: across various spinal regions, including transverse processes, facets, laminae, and spinous processes, in a minimally invasive manner, offering numerous applications in spine surgery [56].
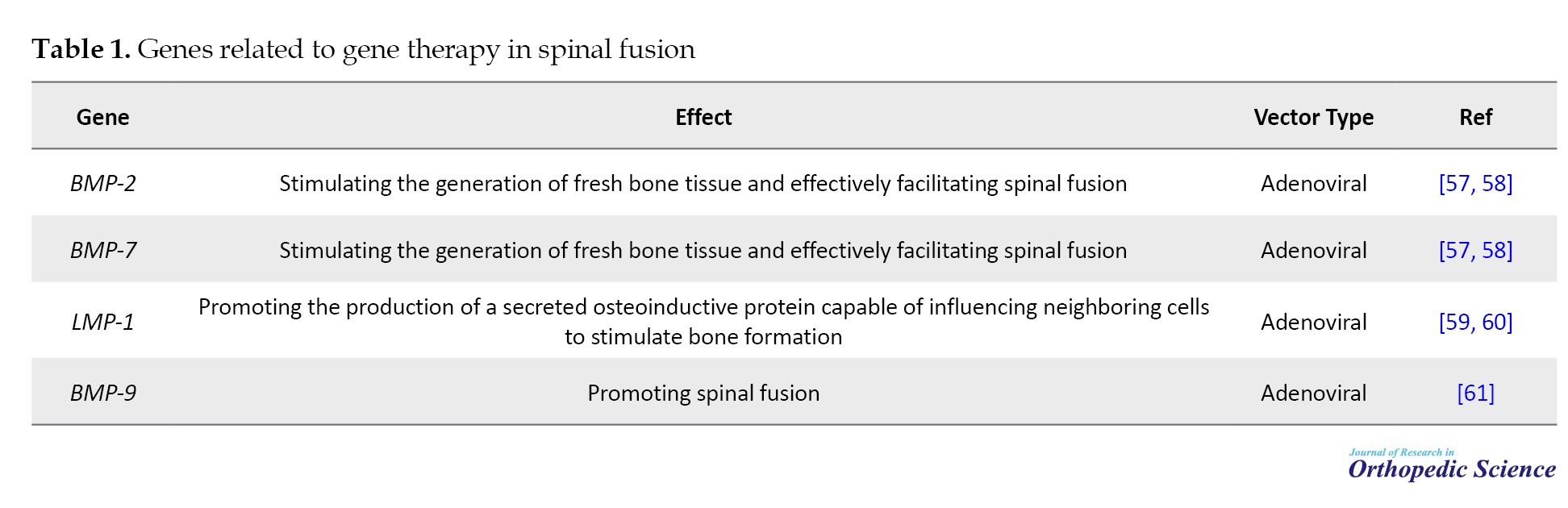
Genes related to gene therapy in spinal fusion are presented in Table 1.
Degeneration of the intervertebral disc
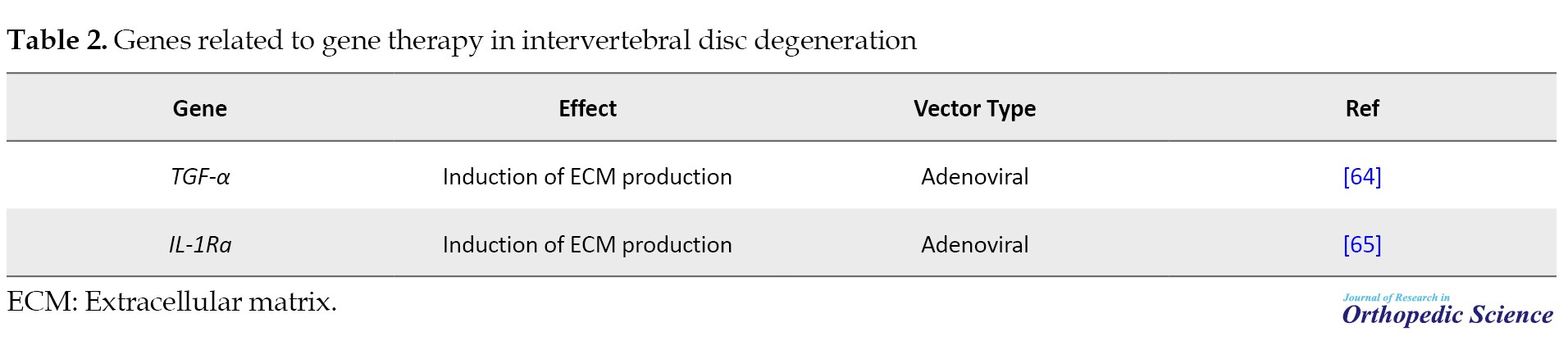
Disc degeneration and related spinal disorders are a significant cause of morbidity, leading to considerable pain and heightened healthcare expenses. Despite extensive clinical research focused on intervertebral discs, current surgical interventions or pharmaceutical treatments do not address the underlying pathology of intervertebral disc degeneration. This degeneration is characterized by weakened or ruptured collagen and proteoglycan structures, reducing water content, and decreased flexibility. Although protein-based agents show promising therapeutic potential with precise targeting, they often face challenges reaching spinal compartments (Table 2) [62, 63].
Bone regeneration
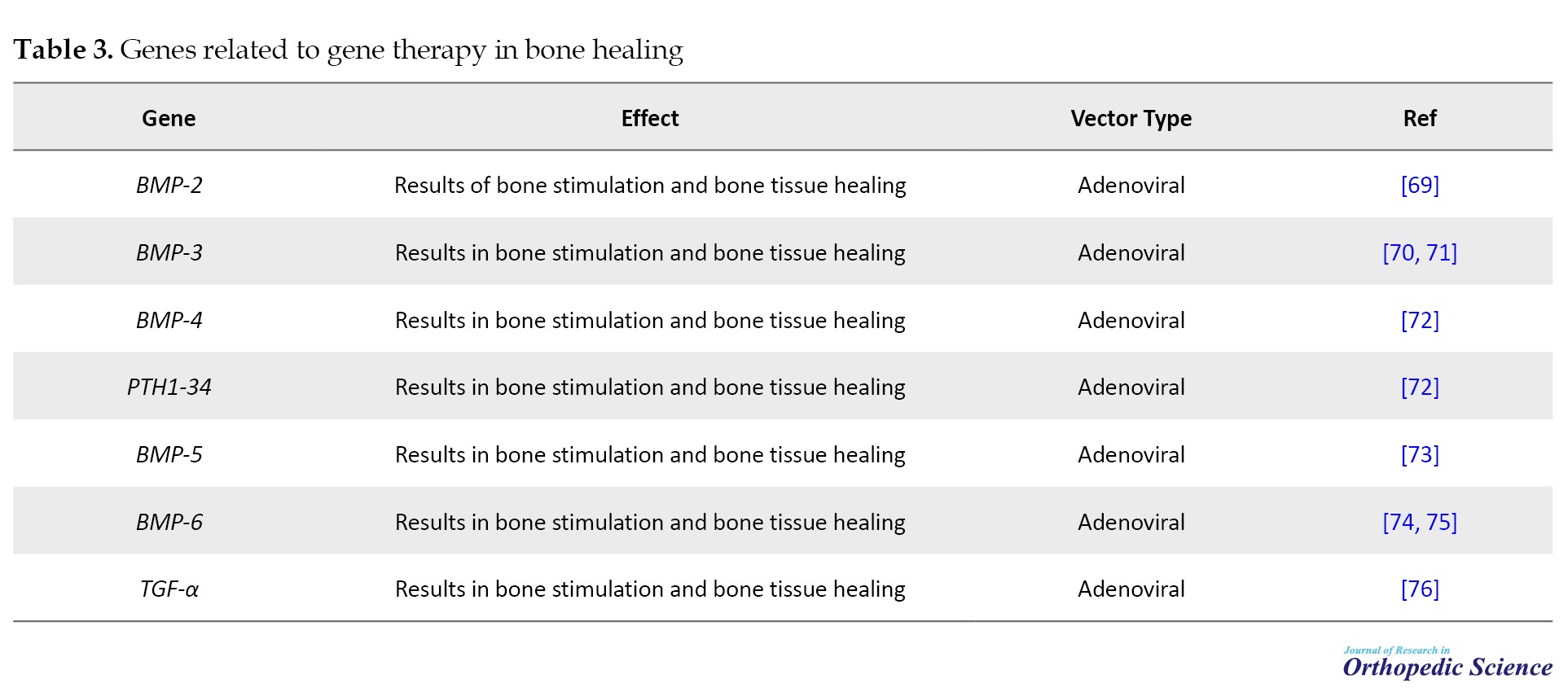
Fracture healing in humans commonly exhibits resilience, requiring only stabilization of the injured area and pain management as medical measures. However, inadequate fracture healing is associated with chronic pain and prolonged mobility limitations, often necessitating surgical intervention. External fixation devices can stabilize fractures prone to inadequate healing; however, insufficient bone at the defect site may lead to structural instability and, in some cases, infection and bone erosion. Although bone grafts are another option, they carry the risk of infection and may not provide sufficient bone for specific applications, making contouring difficult. Microsurgical transfer of free bone grafts, including attached soft tissue and blood vessels, can help reduce the risk of infection; however, it is a complex and specialized procedure with an elevated risk of complications. As a result, modern orthopedic practice often lacks an effective treatment for fractures prone to poor healing tendencies (Table 3) [66-68].
Arthritis
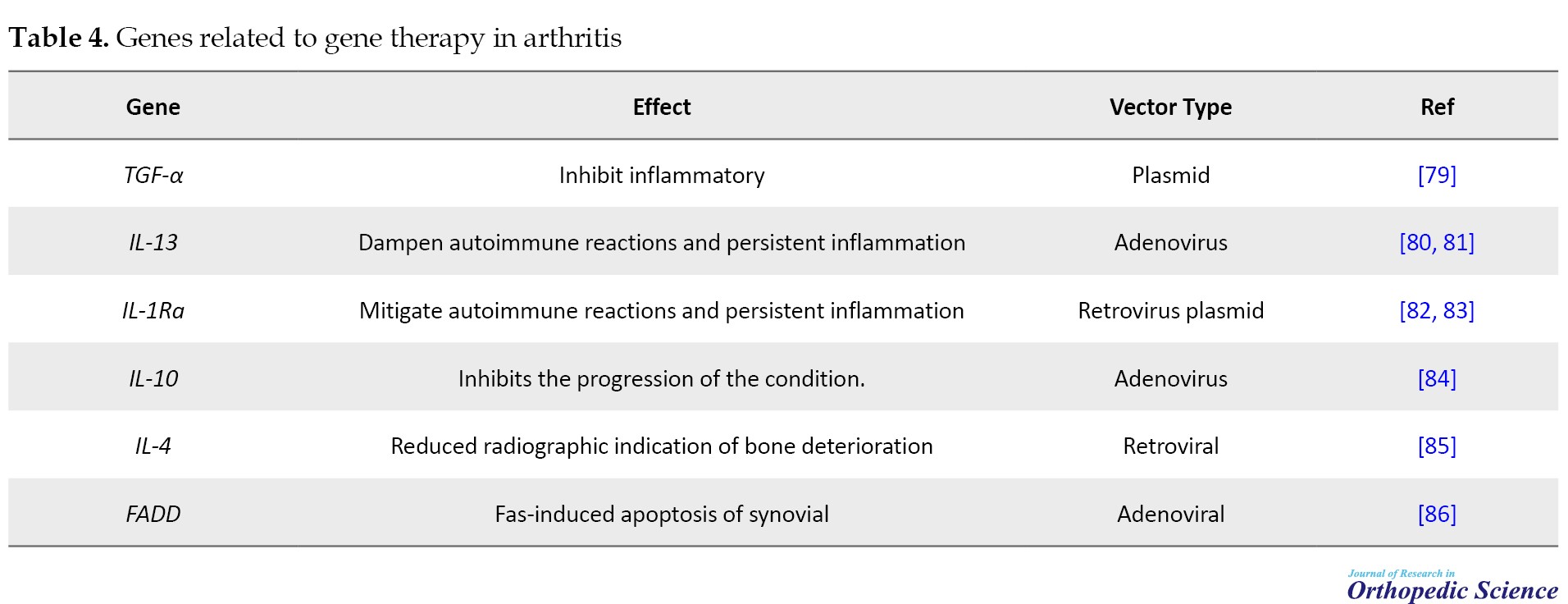
Gene therapy has emerged as a prospective tactic for providing continuous therapeutic concentrations of anti-arthritic gene products to afflicted joints. Viral and non-viral vectors can be used to convey these genes directly into the body (in vivo) or to cells outside the body before re-implantation (ex vivo). Encouraging preclinical outcomes have been attained through implementing these methodologies in diverse animal models of arthritis. Advancements in crafting gene therapies for arthritis have been swift, instilling confidence to enhance the management of this category of ailments (Table 4) [77, 78].
Soft-tissue healing
Injuries to musculoskeletal tissues, such as ligaments and tendons, are prevalent; however, these tissues do not always undergo optimal healing. In healthy individuals, wound healing and tissue repair typically involve multiple stages. Following birth, this process commences with an inflammatory response, upon which subsequent stages are predicted. While wound healing normally restores the injured site, it does not achieve tissue regeneration, which is particularly relevant for mechanically active tissues, such as ligaments and tendons. The complex interplay between mechanical forces and biological processes significantly influences the healing process’s effectiveness. Moreover, host biology is affected by factors such as age, sex, genetics, and tissue history, all of which can influence the healing process [87, 88]. However, their clinical delivery poses significant challenges. In recent years, considerable progress has been made to explore whether gene therapy can overcome these limitations. These encouraging outcomes suggest that this innovative gene therapy approach holds promising potential for advancing soft tissue healing in the foreseeable future (Table 5).
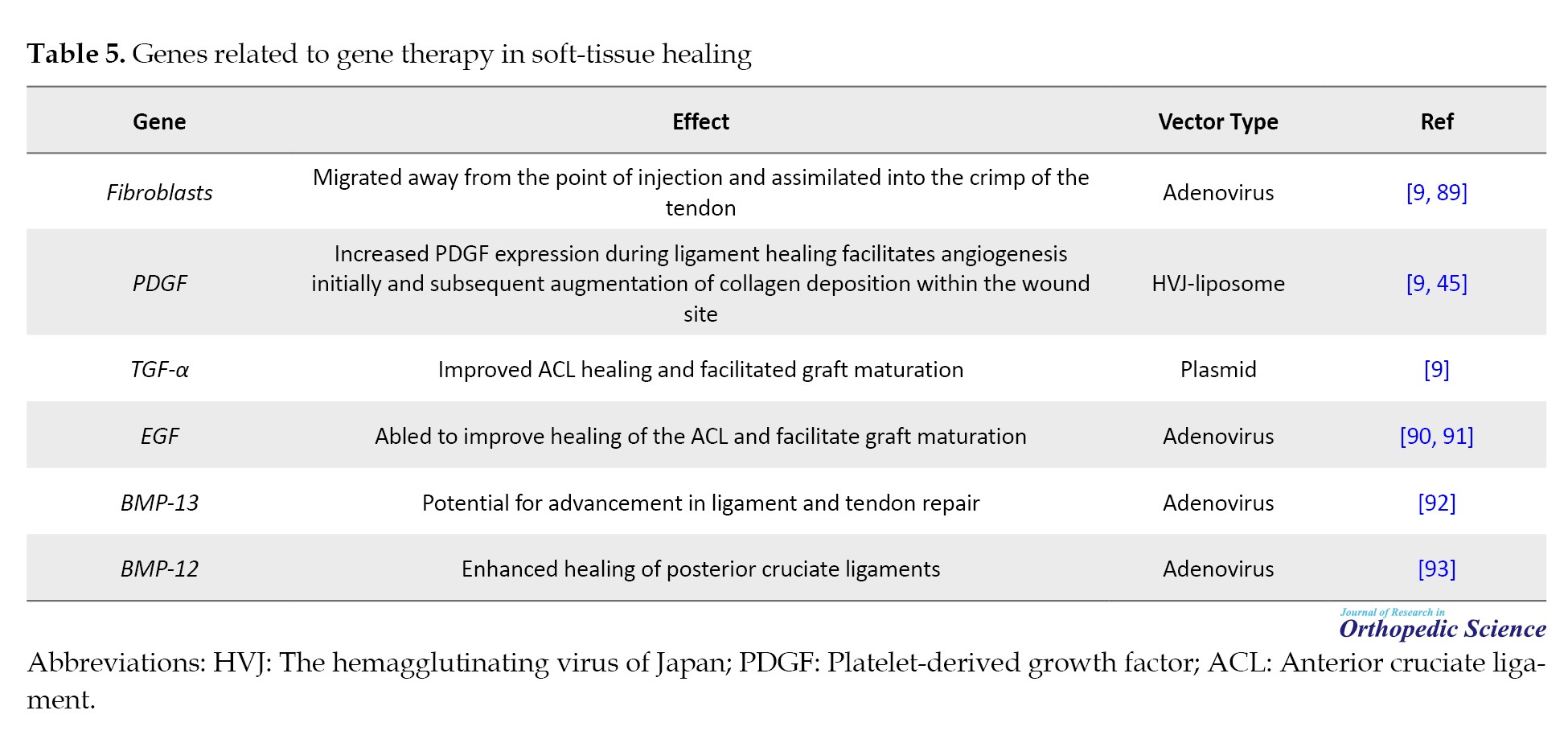
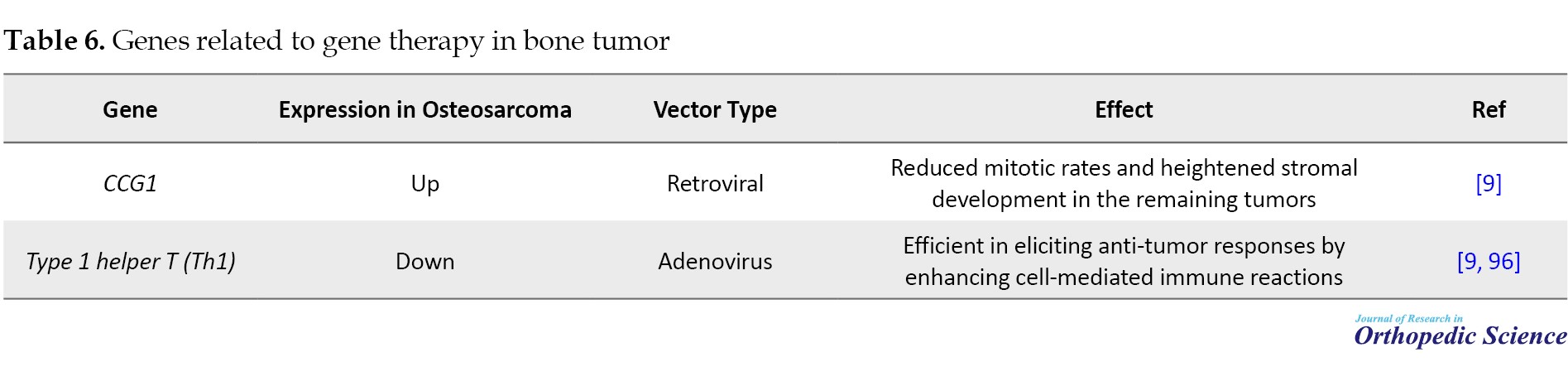
Bone tumor
The traditional treatment for osteosarcoma is aggressive, with relatively modest success rates, and a significant proportion of cases experience relapse. Moreover, traditional treatments for metastatic osteosarcoma primarily focus on palliative care, and metastatic osteosarcoma invariably leads to mortality. As a result, metastatic osteosarcoma is a plausible candidate for gene therapy (Table 6) [94, 95].
Gaucher disease
Gaucher disease, characterized by glycolipid storage dysfunction, arises from numerous mutations in the glucocerebrosidase gene. Approximately 80% of patients with Gaucher exhibit osseous complications, manifesting as bone loss, osteosclerosis, osteonecrosis, and impaired remodeling. Consequential bone loss heightens skeletal fragility, often culminating in pathological fractures that exhibit slow healing tendencies and commonly result in non:union: or mal:union: [97, 98]. The elucidation of the cDNA for the human glucocerebrosidase gene has ushered in the prospect of gene therapy for Gaucher disease. Investigations have illustrated that introducing this gene into fibroblasts from patients with Gaucher syndrome can rectify this defect. In mice, the retroviral transduction of hematopoietic stem cells has successfully achieved sustained glucocerebrosidase gene expression. However, translating this approach into clinical practice requires bone marrow ablation followed by autologous bone marrow transplantation, which poses a significant procedural challenge. Researchers have explored alternative strategies for targeting circulating hematopoietic progenitor cells expressing the CD34 marker to overcome these obstacles. These progenitor cells can be harvested from peripheral blood, genetically modified ex vivo, and reintroduced into the patient through intravenous infusion. Moreover, inherited genetic disorders caused by collagen gene mutations, including chondrodysplasia, Stickler syndrome, and spondyloepiphyseal dysplasia, are candidates for gene therapy. Current efforts aim to optimize these innovative techniques for treating such conditions effectively [9, 99-101].
Disorders affecting nerves and muscles
Gene therapy holds immense promise for revolutionizing the treatment of nerve and muscular injuries. Conditions such as amyotrophic lateral sclerosis and spinal muscular atrophy, which are characterized by progressive paralysis and frequently result in premature mortality, may benefit from novel therapeutic approaches. Although neurotrophic factors have been proposed as potential treatments for these disorders, their clinical use as injected recombinant proteins faces challenges, including toxicity and limited availability. However, research has shown that adenovirus-mediated gene transfer of NT-3 offers significant promise for addressing motor neuron diseases. For instance, intramuscular delivery of this construct demonstrated substantial therapeutic effectiveness in a mouse model of progressive motor neuronopathy [102]. A recent laboratory study introduced a tetracycline-regulated construct encoding nerve growth factor (NGF), demonstrating its dose-dependent modulation and responsiveness to the tetracycline analog doxycycline. These results provide a foundation for future research exploring regulated neurotrophin delivery in animal models of neurodegenerative diseases and nerve injury. Ischemic peripheral neuropathy (IPN) is a common and irreversible complication of lower-extremity vascular insufficiency. Current research efforts aim to evaluate the potential of gene therapy to prevent and/or reverse IPN. In a rabbit model, intramuscular delivery of naked DNA encoding VEGF during hindlimb ischemia effectively prevented a significant decline in motor and sensory nerve functions, facilitating rapid nerve recovery. This positive outcome was partly attributed to improved hindlimb perfusion. Furthermore, the discovery of functional VEGF receptor expression in Schwann cells indicated a direct role for VEGF in maintaining neural integrity [103]. These discoveries represent a novel approach to addressing IPN. The diminishment or alteration of the survival of motor neuron 1 (SMN1) gene results in decreased levels of intracellular survival motor neuron protein, likely contributing to the initiation of spinal muscular atrophy. This effect may occur through potential disruption of spliceosome assembly [104].
Rotator cuff tears
Rotator cuff tears represent frequent soft tissue injuries, often necessitating surgical intervention. Surgical repair of tendon tears markedly enhances pain relief and functional outcomes [105-107]. Previous studies have revealed that up to half of these tears do not heal, as confirmed by ultrasound or magnetic resonance imaging (MRI) [108-110]. Early efforts to improve tendon healing focused on strengthening the repair using more robust suture materials and knots and restoring the rotator cuff’s anatomical footprint using double-row repair techniques. More recent research has focused on the biological augmentation of the healing process [111, 112]. Tissue engineering is an interdisciplinary domain that applies scientific principles to develop living tissues to replace, repair, or enhance diseased tissues [113, 114]. Gene therapy involves the transfer of a specific gene into a cell, prompting the cell to produce a particular protein. The advantage of gene therapy combined with a tissue engineering approach for healing lies in the physician’s ability to select growth factors that play crucial roles in tendon healing. Ideally, enhancing the current repair technique would result in improved tendon healing and, consequently, better clinical outcomes [45, 115, 116].PDGF- is pivotal in accelerating and enhancing tissue healing by facilitating several critical processes. These include chemotaxis, fibroblast proliferation, induction of extracellular matrix components such as fibronectin, and revascularization. Numerous studies have demonstrated that PDGF promotes DNA and matrix synthesis in tendon cells while enhancing the expression of cell-surface integrins, which are essential for tendon repair [117]. IGF-1 also boosts reparative mechanisms by augmenting DNA, collagen, and glycosaminoglycan synthesis. Studies conducted in controlled laboratory settings and within living organisms have clarified IGF-1’s capacity to reduce inflammation and concurrently promote cellular proliferation, collagen generation, and DNA levels [118, 119].
Conclusion
Gene therapy is a promising approach for treating various orthopedic conditions, with research showing feasibility in laboratory and initial clinical trials. Understanding the molecular genetics of skeletal disorders has led to innovative treatments involving the introduction of specific genes into patient cells to influence bone repair and regeneration. This method can potentially manage genetic disorders, such as osteogenesis imperfecta, chronic conditions, such as arthritis, and injuries, such as bone and cartilage damage. Animal studies have shown positive results in osteoarthritis, bone healing, and ligament healing. However, challenges remain in identifying optimal cellular targets, therapeutic genes, and safe delivery methods. Future research should aim to understand the genes and signaling pathways involved in bone cell growth, identify more effective therapeutic genes, and explore combining genes for better outcomes. Non-viral vectors have emerged as alternatives to viral vectors. The integration of gene therapy with existing treatments, such as protein therapy and tissue engineering, is also being explored. Advances in genetic markers, genomics, and proteomics will help identify treatment targets, and molecular imaging technology will aid in studying the role of expressed molecules, leading to further progress in this field.
Ethical Considerations
Compliance with ethical guidelines
There were no ethical considerations to be considered in this research.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors contributed equally to the conception and design of the study, data collection and analysis, interception of the results and drafting of the manuscript. Each author approved the final version of the manuscript for submission.
Conflict of interest
The authors declared no conflict of interest.
References
Genes act as fundamental building blocks of heredity, governing individuals’ physical traits and functional characteristics. At birth, individuals inherit a predetermined genetic blueprint, which, when coupled with environmental influences, dictates susceptibility to various disease conditions. Gene therapy has emerged as a strategy for correcting aberrant genes associated with disease development, involving the transfer and activation of genes within individuals for therapeutic purposes [1, 2]. Gene therapy consists of the delivery of genes to individuals with a therapeutic aim. Human gene transfer can be achieved through ex vivo and in vivo approaches. Ex vivo gene therapy involves the transfer of genes into cells outside the body, typically in a tissue culture environment. Subsequently, genetically modified cells are reintroduced into the host organism. In contrast, the in vivo approach entails the direct transfer of genes into specific somatic cells of the host organism in its native environment [3]. Ex vivo techniques are notably more intricate but offer relatively higher safety profiles. Additionally, the ex vivo approach provides an opportunity to select cells expressing the desired genes at elevated levels in vitro. In contrast, in vivo gene delivery techniques are more straightforward from a technical standpoint and are currently more widely employed. The application of gene therapy to germline cells, initially envisioned as a remedy for hereditary genetic conditions, raises ethical dilemmas owing to the far-reaching implications of modifications, impacting all future generations and potentially evoking concerns related to eugenics. As a result, while experimental somatic cell gene therapy represents a relatively emerging domain, substantial advancements have been made in the last decade. The widespread recognition of the potential of gene therapy to transform medical practices in the 21st century is evident [4]. This review underscores this innovative approach’s utilization and prospective benefits in tackling orthopedic disorders.
Strategies for Gene Therapy
Gene therapy can be categorized into two main branches: Somatic cells and germline cells. It is crucial to highlight that all clinical trials conducted thus far exclusively examined somatic cell gene therapy [5, 6]. In somatic gene therapy, a plethora of tissues are available for consideration. Additionally, once the target tissue is identified, selecting the target cell in that tissue presents a considerable challenge, often surpassing the complexity of choosing the target tissue itself. The decision regarding which cell to target frequently depends on the method employed for vector delivery [7]. In gene therapy, the synthesis of a functional protein involves several sequential processes. First, exogenous complementary DNA (cDNA) must traverse the cell membrane, evade degradation within the lysosomes, and subsequently access the nucleus for transcription. After transcription, the resulting messenger ribonucleic acid (mRNA) is translated into amino acids, culminating in the production of the intended peptide or protein [8]. Gene therapy holds promise for delivering growth factors in a biologically active manner because the protein is synthesized endogenously in the body. This can result in more accurate post-translational modifications and the formation of tertiary structures with ligands that are more readily recognizable, thereby augmenting their ability to bind to cell surface receptors compared with recombinant proteins [9, 10].
Gene Delivery Vectors
For gene expression to occur, the transferred DNA must successfully enter the host cell’s nucleus, where it can integrate into the host cell’s chromosomes or remain in episomes. Introduction is the process of gene transfer using a viral vector. Viral vectors employed in human clinical trials include retroviruses, adenoviruses, adeno-associated viruses, lentiviruses, and HSVs [11, 12]. The Moloney murine leukemia retrovirus is one of the most well-established viral vectors used in gene therapy. The essential attributes of any viral vector pertinent to gene therapy include simplicity, cost-effectiveness of production, potential for evoking immune responses, integration into the host genome, titer, and safety profile [13]. The specific advantages and limitations of each available viral vector system have been comprehensively examined in the literature [14].
Viral Vectors
Vectors derived from retroviruses
Retroviruses are RNA viruses that replicate via an intermediate step involving DNA [3]. The primary advantage of retroviral vectors is their exceptional efficiency in transferring genes into actively dividing cells. This level of accuracy and durability of gene transfer is unmatched by other types of viruses [15]. Retroviral vectors have a substantial cloning capacity, accommodating up to 8 kilobases (kb) of genetic material, and can be manufactured in significant quantities with relative ease for clinical applications. However, their utility is constrained by factors such as a limited host range, inadequate transduction of non-dividing cells, and potential risk of insertional events that trigger tumorigenesis [16]. These delivery systems can transduce osteoblasts, bone marrow stromal cells, and muscle-derived stem cells, positioning them as promising candidates for skeletal gene therapy [17-19]. Investigators found that periosteal mesenchymal stem cells, engineered to express bone morphogenetic protein (BMP)-7 via retroviral vector manipulation, markedly improve the healing process of critical-sized defects. Researchers in a mouse calvarial model noted synergistic advantages in bone regeneration by co-implanting muscle-derived stem cells, genetically modified ex vivo using retroviral vectors to produce BMP-2/4 and vascular endothelial growth factor (VEGF) [20, 21].
Vectors derived from adenovirus
Adenoviruses, DNA viruses featuring a double-stranded genome approximately 35 kb in length, have been extensively evaluated in both preclinical and clinical trials for gene therapy applications [22]. These vectors can accommodate approximately eight kb of genetic material within an expression cassette. However, newer “gutless” adenoviral vectors can accommodate significantly larger DNA sequences [23]. Adenovirus vectors exhibit remarkable efficiency, facilitating their production in significant quantities and resulting in elevated levels of expression following transduction. Furthermore, they demonstrate the capacity to transfer genes to replicating and non-replicating cells [3]. The primary hurdle associated with using adenoviruses in gene therapy pertains to the immune response elicited by the host, which restricts transgene expression in animals with intact immune systems [10]. Adenoviral vectors expressing lacZ and transforming growth factor β1 (TGF-β1) efficiently transduced osteoblasts and osteoclasts, resulting in notable alterations within the epiphyseal plate [24, 25].
Vectors derived from herpes simplex virus (HSV)
HSV vector, known for its remarkable DNA virus and infectivity, remains latent in nerve cells. However, its extensive genome and cytotoxic properties make it less favorable for consideration as a vector [26]. Researchers have recently employed a second-generation, low-toxicity HSV vector to deliver an interleukin-1 receptor antagonist (IL-1Ra) gene and a soluble tumor necrosis factor-α (TNF-α) receptor gene. This approach significantly reduced arthritis symptoms, primarily through IL-1Ra [27].
Vectors derived from lentiviral
For the most part, lentiviral vectors are derived from human immunodeficiency virus (HIV). Consequently, prioritizing safety is crucial when employing this gene delivery platform. Lentiviral vectors engineered from HIV demonstrate efficient transduction of human macrophages and primary tissues, such as the brain and muscle [28, 29]. Despite persistent safety concerns, the capacity of lentiviral vectors to infect non-dividing osteogenic cells, their compatibility with osteoblast-specific promoters, and their reduced propensity to induce gene silencing or activate host cell genes strongly indicate that these vectors exert a significant influence on future skeletal gene therapy strategies.
Vectors derived from adeno-associated virus
Recently, recombinant adeno-associated viral (AAV) vectors have emerged as promising substitutes for adenoviral and retroviral vectors for gene therapy. AAV vectors demonstrate non-cytotoxic properties, exceptional safety profiles, and the ability to deliver genes to non-dividing cells. They can be integrated explicitly into the 19th chromosome, ensuring targeted gene insertion. Furthermore, they enable prolonged and consistent gene expression and can be produced at high titers, facilitating their application using in vivo methods [1]. The researchers utilized AAV vectors to deliver marker genes to the arthritic knees of mice overexpressing TNF-α using in vivo techniques. AAV has demonstrated effectiveness as a vector and is expected to be applied in a broader spectrum of clinical treatments [30].
Vectors derived from non-viral
There has been a push for developing non-viral delivery systems owing to concerns regarding safety, immunogenicity, and production limitations associated with viral vectors. These systems involve a combination of genes (DNA) and various chemical formulations. The technique referred to as transfection illustrates a form of gene transfer that does not include viruses. Non-viral delivery systems encompass a diverse range of materials, such as plasmids, peptides, positively charged liposomes, DNA complexes with ligands targeting particular cell receptors to enhance cellular uptake, and Gene-gun technology, which employs gold-coated particles loaded with DNA and introducing them into cells through high-speed bombardment [31-33]. However, their effectiveness typically falls short of that of viral vectors. Non-viral delivery systems demonstrate lower efficiency than viral techniques because no intrinsic biological mechanism is found to integrate the desired DNA material into the genome. These approaches can be classified as physical, mechanical, or chemical approaches. The researchers injected plasmid DNA encoding TGF-α1 directly into the muscles of mice with streptococcal cell wall-induced arthritis. They observed substantial suppression of chronic diseases, which was marked by reduced inflammation at the peak of the acute phase. Additionally, cartilage, bone damage, and pannus formation decrease is observed during the chronic phase [34].
Clinical Applications
Cartilage repair
Impairment of adult articular cartilage frequently results in early-onset arthritis, mainly due to the tissue’s limited regenerative ability. This limitation can be attributed to insufficient availability of stem cells, inadequate vascularization, and low cellular turnover [35]. Numerous methods have been devised to enhance the healing of articular cartilage [36, 37]. Mason et al. used a retroviral vector to genetically modify mesenchymal stem cells by introducing a BMP-7 gene. These modified cells were transplanted onto a polyglycolic acid scaffold to a rabbit osteochondral defect [38, 39]. They demonstrated significantly improved healing of the articular defect compared to control groups at both 8 and 12 weeks post-implantation. More recently, researchers have investigated the effects of in vitro gene transfer of insulin-like growth factor-1 (IGF-1), BMP-2, and TGF-β to rabbit articular chondrocytes [39]. Studies indicate that BMP-7 also stimulates the chondrogenic differentiation of precursor cells derived from the periosteum. Research has shown that incorporating periosteal cells genetically modified to express BMP-7 or sonic hedgehog cDNAs improves the healing of osteochondral defects in rabbits [40]. Given their limited intrinsic capacity for cartilage repair and remodeling, gene therapy cells have often been integrated with diverse scaffolds to replicate the architecture of cartilage tissues. This approach has shown promising results in cartilage repair in rabbits, mainly using periosteal mesenchymal stem cells transfected with BMP-7 and sonic hedgehog genes [41].
Meniscus
Various methods, such as sutures, arrows, and staples, have been devised to conserve the menisci. Nonetheless, tears located solely in the vascularized outer third of the meniscus exhibit healing potential [42]. Preconditioning meniscus allografts using viral vectors expressing growth factors holds promise for expediting graft healing and restructuring while mitigating immunogenic responses. The rationale for employing gene-based approaches to preserve and repair the articular cartilage can be extended to the meniscus. Meniscal cells are responsive to adenoviral and retroviral transduction. Specifically, the delivery of TGF-β1 cDNA into these cells in monolayer culture resulted in a significant increase in proteoglycan and collagen production, with no changes detected in the cells’ collagen phenotype [43-47].
Osteoporosis
Osteoporosis leads to reduced bone density and osteopenia [48]. Osteoporosis manifests as two distinct types. Type 1 osteoporosis is characterized by escalated osteoclastogenesis stemming from estrogen depletion, whereas type 2 is characterized by diminished osteogenesis originating from aging marrow stem cells. Gene therapy for type 2 osteoporosis can be implemented using ex vivo methods involving the transduction of marrow stem cells from osteoporotic donors with adenoviral vectors encoding the BMP-2 gene. This potent growth factor promotes bone formation. Studies have shown that genetically engineered cells significantly enhance osteogenic activity in vivo [49]. Systemic intravenous delivery of adenoviral or AAV vectors containing osteoprotegerin (OPG) cDNA results in elevated levels of OPG in circulation, thereby eliciting a sustained anti-osteoporotic effect in mice [50, 51].
Osteopetrosis
Osteopetrosis, a genetic disorder characterized by excessive bone formation and bone marrow obliteration, presents an opposite phenotype to osteoporosis. Surplus bone formation in osteopetrosis is caused by decreased osteoclastogenesis, which is associated with genetic abnormalities affecting the colony-stimulating factor 1 (CSF-1) gene. Gene therapy offers potential by introducing marrow stem cells modified to overexpress the CSF-1 gene, thereby stimulating heightened osteoclastogenesis [52-54].
Spinal fusion
Spinal fusion is a common procedure in spinal surgery, frequently requiring internal fixation devices for temporary stabilization. However, achieving enduring stability requires a successful bone fusion. Nonetheless, the failure rate to achieve robust bone fusion can reach 45%. Although autogenous bone grafts are efficacious, they are constrained in volume and can induce considerable morbidity at the donor site. Research indicated that the morbidity rate associated with harvesting autogenous iliac crest bone grafts may reach 30%. Allograft bone carries risks of antigenicity and disease transmission. Additionally, alloplastic materials are associated with higher infection rate and extrusion and inferior biomechanical properties [55]. Extensive animal studies have demonstrated the exceptional effectiveness of BMPs in enhancing bone formation at fusion sites, offering strong support for advancing BMP gene therapy to clinical applications. Unlike systemic gene therapy, local gene therapy for spinal fusion is less complex because it requires sustained gene expression for only a brief period, potentially less than a week, to trigger the endochondral osteoinduction cascade. Although several hurdles must be overcome before this innovative approach can be clinically deployed, local gene therapy shows promise as a more favorable method of osteoinduction than the administration of pharmacological doses of recombinant or extracted osteoinductive proteins in spinal surgery. With further refinement, BMP gene therapy stands poised to facilitate bony :union: across various spinal regions, including transverse processes, facets, laminae, and spinous processes, in a minimally invasive manner, offering numerous applications in spine surgery [56].
Genes related to gene therapy in spinal fusion are presented in Table 1.

Degeneration of the intervertebral disc
Disc degeneration and related spinal disorders are a significant cause of morbidity, leading to considerable pain and heightened healthcare expenses. Despite extensive clinical research focused on intervertebral discs, current surgical interventions or pharmaceutical treatments do not address the underlying pathology of intervertebral disc degeneration. This degeneration is characterized by weakened or ruptured collagen and proteoglycan structures, reducing water content, and decreased flexibility. Although protein-based agents show promising therapeutic potential with precise targeting, they often face challenges reaching spinal compartments (Table 2) [62, 63].

Bone regeneration
Fracture healing in humans commonly exhibits resilience, requiring only stabilization of the injured area and pain management as medical measures. However, inadequate fracture healing is associated with chronic pain and prolonged mobility limitations, often necessitating surgical intervention. External fixation devices can stabilize fractures prone to inadequate healing; however, insufficient bone at the defect site may lead to structural instability and, in some cases, infection and bone erosion. Although bone grafts are another option, they carry the risk of infection and may not provide sufficient bone for specific applications, making contouring difficult. Microsurgical transfer of free bone grafts, including attached soft tissue and blood vessels, can help reduce the risk of infection; however, it is a complex and specialized procedure with an elevated risk of complications. As a result, modern orthopedic practice often lacks an effective treatment for fractures prone to poor healing tendencies (Table 3) [66-68].

Arthritis
Gene therapy has emerged as a prospective tactic for providing continuous therapeutic concentrations of anti-arthritic gene products to afflicted joints. Viral and non-viral vectors can be used to convey these genes directly into the body (in vivo) or to cells outside the body before re-implantation (ex vivo). Encouraging preclinical outcomes have been attained through implementing these methodologies in diverse animal models of arthritis. Advancements in crafting gene therapies for arthritis have been swift, instilling confidence to enhance the management of this category of ailments (Table 4) [77, 78].

Soft-tissue healing
Injuries to musculoskeletal tissues, such as ligaments and tendons, are prevalent; however, these tissues do not always undergo optimal healing. In healthy individuals, wound healing and tissue repair typically involve multiple stages. Following birth, this process commences with an inflammatory response, upon which subsequent stages are predicted. While wound healing normally restores the injured site, it does not achieve tissue regeneration, which is particularly relevant for mechanically active tissues, such as ligaments and tendons. The complex interplay between mechanical forces and biological processes significantly influences the healing process’s effectiveness. Moreover, host biology is affected by factors such as age, sex, genetics, and tissue history, all of which can influence the healing process [87, 88]. However, their clinical delivery poses significant challenges. In recent years, considerable progress has been made to explore whether gene therapy can overcome these limitations. These encouraging outcomes suggest that this innovative gene therapy approach holds promising potential for advancing soft tissue healing in the foreseeable future (Table 5).

Bone tumor
The traditional treatment for osteosarcoma is aggressive, with relatively modest success rates, and a significant proportion of cases experience relapse. Moreover, traditional treatments for metastatic osteosarcoma primarily focus on palliative care, and metastatic osteosarcoma invariably leads to mortality. As a result, metastatic osteosarcoma is a plausible candidate for gene therapy (Table 6) [94, 95].

Gaucher disease
Gaucher disease, characterized by glycolipid storage dysfunction, arises from numerous mutations in the glucocerebrosidase gene. Approximately 80% of patients with Gaucher exhibit osseous complications, manifesting as bone loss, osteosclerosis, osteonecrosis, and impaired remodeling. Consequential bone loss heightens skeletal fragility, often culminating in pathological fractures that exhibit slow healing tendencies and commonly result in non:union: or mal:union: [97, 98]. The elucidation of the cDNA for the human glucocerebrosidase gene has ushered in the prospect of gene therapy for Gaucher disease. Investigations have illustrated that introducing this gene into fibroblasts from patients with Gaucher syndrome can rectify this defect. In mice, the retroviral transduction of hematopoietic stem cells has successfully achieved sustained glucocerebrosidase gene expression. However, translating this approach into clinical practice requires bone marrow ablation followed by autologous bone marrow transplantation, which poses a significant procedural challenge. Researchers have explored alternative strategies for targeting circulating hematopoietic progenitor cells expressing the CD34 marker to overcome these obstacles. These progenitor cells can be harvested from peripheral blood, genetically modified ex vivo, and reintroduced into the patient through intravenous infusion. Moreover, inherited genetic disorders caused by collagen gene mutations, including chondrodysplasia, Stickler syndrome, and spondyloepiphyseal dysplasia, are candidates for gene therapy. Current efforts aim to optimize these innovative techniques for treating such conditions effectively [9, 99-101].
Disorders affecting nerves and muscles
Gene therapy holds immense promise for revolutionizing the treatment of nerve and muscular injuries. Conditions such as amyotrophic lateral sclerosis and spinal muscular atrophy, which are characterized by progressive paralysis and frequently result in premature mortality, may benefit from novel therapeutic approaches. Although neurotrophic factors have been proposed as potential treatments for these disorders, their clinical use as injected recombinant proteins faces challenges, including toxicity and limited availability. However, research has shown that adenovirus-mediated gene transfer of NT-3 offers significant promise for addressing motor neuron diseases. For instance, intramuscular delivery of this construct demonstrated substantial therapeutic effectiveness in a mouse model of progressive motor neuronopathy [102]. A recent laboratory study introduced a tetracycline-regulated construct encoding nerve growth factor (NGF), demonstrating its dose-dependent modulation and responsiveness to the tetracycline analog doxycycline. These results provide a foundation for future research exploring regulated neurotrophin delivery in animal models of neurodegenerative diseases and nerve injury. Ischemic peripheral neuropathy (IPN) is a common and irreversible complication of lower-extremity vascular insufficiency. Current research efforts aim to evaluate the potential of gene therapy to prevent and/or reverse IPN. In a rabbit model, intramuscular delivery of naked DNA encoding VEGF during hindlimb ischemia effectively prevented a significant decline in motor and sensory nerve functions, facilitating rapid nerve recovery. This positive outcome was partly attributed to improved hindlimb perfusion. Furthermore, the discovery of functional VEGF receptor expression in Schwann cells indicated a direct role for VEGF in maintaining neural integrity [103]. These discoveries represent a novel approach to addressing IPN. The diminishment or alteration of the survival of motor neuron 1 (SMN1) gene results in decreased levels of intracellular survival motor neuron protein, likely contributing to the initiation of spinal muscular atrophy. This effect may occur through potential disruption of spliceosome assembly [104].
Rotator cuff tears
Rotator cuff tears represent frequent soft tissue injuries, often necessitating surgical intervention. Surgical repair of tendon tears markedly enhances pain relief and functional outcomes [105-107]. Previous studies have revealed that up to half of these tears do not heal, as confirmed by ultrasound or magnetic resonance imaging (MRI) [108-110]. Early efforts to improve tendon healing focused on strengthening the repair using more robust suture materials and knots and restoring the rotator cuff’s anatomical footprint using double-row repair techniques. More recent research has focused on the biological augmentation of the healing process [111, 112]. Tissue engineering is an interdisciplinary domain that applies scientific principles to develop living tissues to replace, repair, or enhance diseased tissues [113, 114]. Gene therapy involves the transfer of a specific gene into a cell, prompting the cell to produce a particular protein. The advantage of gene therapy combined with a tissue engineering approach for healing lies in the physician’s ability to select growth factors that play crucial roles in tendon healing. Ideally, enhancing the current repair technique would result in improved tendon healing and, consequently, better clinical outcomes [45, 115, 116].PDGF- is pivotal in accelerating and enhancing tissue healing by facilitating several critical processes. These include chemotaxis, fibroblast proliferation, induction of extracellular matrix components such as fibronectin, and revascularization. Numerous studies have demonstrated that PDGF promotes DNA and matrix synthesis in tendon cells while enhancing the expression of cell-surface integrins, which are essential for tendon repair [117]. IGF-1 also boosts reparative mechanisms by augmenting DNA, collagen, and glycosaminoglycan synthesis. Studies conducted in controlled laboratory settings and within living organisms have clarified IGF-1’s capacity to reduce inflammation and concurrently promote cellular proliferation, collagen generation, and DNA levels [118, 119].
Conclusion
Gene therapy is a promising approach for treating various orthopedic conditions, with research showing feasibility in laboratory and initial clinical trials. Understanding the molecular genetics of skeletal disorders has led to innovative treatments involving the introduction of specific genes into patient cells to influence bone repair and regeneration. This method can potentially manage genetic disorders, such as osteogenesis imperfecta, chronic conditions, such as arthritis, and injuries, such as bone and cartilage damage. Animal studies have shown positive results in osteoarthritis, bone healing, and ligament healing. However, challenges remain in identifying optimal cellular targets, therapeutic genes, and safe delivery methods. Future research should aim to understand the genes and signaling pathways involved in bone cell growth, identify more effective therapeutic genes, and explore combining genes for better outcomes. Non-viral vectors have emerged as alternatives to viral vectors. The integration of gene therapy with existing treatments, such as protein therapy and tissue engineering, is also being explored. Advances in genetic markers, genomics, and proteomics will help identify treatment targets, and molecular imaging technology will aid in studying the role of expressed molecules, leading to further progress in this field.
Ethical Considerations
Compliance with ethical guidelines
There were no ethical considerations to be considered in this research.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors contributed equally to the conception and design of the study, data collection and analysis, interception of the results and drafting of the manuscript. Each author approved the final version of the manuscript for submission.
Conflict of interest
The authors declared no conflict of interest.
References
- Giannoudis PV, Tzioupis CC, Tsiridis E. Gene therapy in orthopaedics. Injury. 2006; 37(Suppl 1):S30-40. [DOI:10.1016/j.injury.2006.02.038] [PMID]
- Ma CC, Wang ZL, Xu T, He ZY, Wei YQ. The approved gene therapy drugs worldwide: From 1998 to 2019. Biotechnol Adv. 2020; 40:107502. [DOI:10.1016/j.biotechadv.2019.107502] [PMID]
- Crystal RG. Transfer of genes to humans: Early lessons and obstacles to success. Science. 1995; 270(5235):404-10. [DOI:10.1126/science.270.5235.404] [PMID]
- Marcum JA. From the molecular genetics revolution to gene therapy: Translating basic research into medicine. J Lab Clin Med. 2005; 146(6):312-6. [DOI:10.1016/j.lab.2005.07.009] [PMID]
- Evans CH, Ghivizzani SC, Herndon JH, Robbins PD. Gene therapy for the treatment of musculoskeletal diseases. J Am Acad Orthop Surg. 2005; 13(4):230-42. [DOI:10.5435/00124635-200507000-00003] [PMID]
- Kaji EH, Leiden JM. Gene and stem cell therapies. JAMA. 2001; 285(5):545-50. [DOI:10.1001/jama.285.5.545] [PMID]
- Hannallah D, Peterson B, Lieberman JR, Fu FH, Huard J. Gene therapy in orthopaedic surgery. Instr Course Lect. 2003; 52:753-68. [PMID]
- Southwood LL, Frisbie DD, Kawcak CE, McIlwraith CW. Delivery of growth factors using gene therapy to enhance bone healing. Vet Surg. 2004; 33(6):565-78. [DOI:10.1111/j.1532-950x.2004.04080.x] [PMID]
- Chen Y. Orthopedic applications of gene therapy. J Orthop Sci. 2001; 6(2):199-207. [DOI:10.1007/s007760100072] [PMID]
- Niyibizi C, Baltzer A, Lattermann C, Oyama M, Whalen JD, Robbins PD, et al. Potential role for gene therapy in the enhancement of fracture healing. Clin Orthop Relat Res. 1998; (355 Suppl):S148-53. [DOI:10.1097/00003086-199810001-00016] [PMID]
- Naldini L, Blömer U, Gallay P, Ory D, Mulligan R, Gage FH, et al. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996; 272(5259):263-7. [DOI:10.1126/science.272.5259.263] [PMID]
- Buchschacher GL Jr, Panganiban AT. Human immunodeficiency virus vectors for inducible expression of foreign genes. J Virol. 1992; 66(5):2731-9. [DOI:10.1128/jvi.66.5.2731-2739.1992] [PMID] [PMCID]
- Steinert AF, Palmer GD, Evans CH. Gene therapy in the musculoskeletal system. Curr Opin Orthop. 2004; 15(5):318-24. [DOI:10.1097/01.bco.0000136128.77171.0b]
- Thomas CE, Ehrhardt A, Kay MA. Progress and problems with the use of viral vectors for gene therapy. Nat Rev Genet. 2003; 4(5):346-58. [DOI:10.1038/nrg1066] [PMID]
- Li H, Zou X, Bünger C. Gene therapy and spinal disorders. Int Orthop. 2001; 25(1):1-4. [DOI:10.1007/s002640000207] [PMID] [PMCID]
- Wu X, Li Y, Crise B, Burgess SM. Transcription start regions in the human genome are favored targets for MLV integration. Science. 2003; 300(5626):1749-51. [DOI:10.1126/science.1083413] [PMID]
- Dodig M, Tadic T, Kronenberg MS, Dacic S, Liu YH, Maxson R, et al. Ectopic Msx2 overexpression inhibits and Msx2 antisense stimulates calvarial osteoblast differentiation. Dev Biol. 1999; 209(2):298-307. [DOI:10.1006/dbio.1999.9258] [PMID]
- Oreffo RO, Virdi AS, Triffitt JT. Retroviral marking of human bone marrow fibroblasts: In vitro expansion and localization in calvarial sites after subcutaneous transplantation in vivo. J Cell Physiol. 2001; 186(2):201-9. [DOI:10.1002/1097-4652(200102)186:23.0.CO;2-B] [PMID]
- Fiorellini JP, Buser D, Riley E, Howell TH. Effect on bone healing of bone morphogenetic protein placed in combination with endosseous implants: A pilot study in beagle dogs. Int J Periodontics Restorative Dent. 2001; 21(1):41-7. [PMID]
- Breitbart AS, Grande DA, Mason JM, Barcia M, James T, Grant RT. Gene-enhanced tissue engineering: Applications for bone healing using cultured periosteal cells transduced retrovirally with the BMP-7 gene. Ann Plast Surg. 1999; 42(5):488-95. [DOI:10.1097/00000637-199905000-00005] [PMID]
- Peng H, Wright V, Usas A, Gearhart B, Shen HC, Cummins J, et al. Synergistic enhancement of bone formation and healing by stem cell-expressed VEGF and bone morphogenetic protein-4. J Clin Invest. 2002; 110(6):751-9. [DOI:10.1172/JCI15153] [PMID] [PMCID]
- Culver KW. Gene therapy: A primer for physicians. New Rochelle: Mary Ann Liebert; 1996. [Link]
- Haecker SE, Stedman HH, Balice-Gordon RJ, Smith DB, Greelish JP, Mitchell MA, et al. In vivo expression of full-length human dystrophin from adenoviral vectors deleted of all viral genes. Hum Gene Ther. 1996; 7(15):1907-14. [DOI:10.1089/hum.1996.7.15-1907] [PMID]
- Mehrara BJ, Saadeh PB, Steinbrech DS, Dudziak M, Spector JA, Greenwald JA, et al. Adenovirus-mediated gene therapy of osteoblasts in vitro and in vivo. J Bone Miner Res. 1999; 14(8):1290-301. [DOI:10.1359/jbmr.1999.14.8.1290] [PMID]
- van Griensven M, Lobenhoffer P, Barke A, Tschernig T, Lindenmaier W, Krettek C, et al. Adenoviral gene transfer in a rat fracture model. Lab Anim. 2002; 36(4):455-61. [DOI:10.1258/002367702320389134] [PMID]
- Singh S, Jindal D, Khanna R. Can serum MMP-3 diagnose early knee osteoarthritis? J Orthop. 2023; 38:42-46. [DOI:10.1016/j.jor.2023.02.014] [PMID] [PMCID]
- Li W, Sun J, Feng SL, Wang F, Miao MZ, Wu EY, et al. Intra-articular delivery of AAV vectors encoding PD-L1 attenuates joint inflammation and tissue damage in a mouse model of rheumatoid arthritis. Front Immunol. 2023; 14:1116084. [DOI:10.3389/fimmu.2023.1116084] [PMID] [PMCID]
- Buck AM, Deveau TM, Henrich TJ, Deitchman AN. Challenges in HIV-1 latent reservoir and target cell quantification in CAR-T cell and other lentiviral gene modifying HIV cure strategies. Viruses. 2023; 15(5):1126. [DOI:10.3390/v15051126] [PMID] [PMCID]
- Howe G, Wasmuth M, Emanuelle P, Massaro G, Rahim AA, Ali S, et al. Engineering an autonucleolytic mammalian suspension host cell line to reduce DNA impurity levels in serum-free lentiviral process streams. ACS Synth Biol. 2024; 13(2):466-73. [DOI:10.1021/acssynbio.3c00682] [PMID] [PMCID]
- Schwarz EM. The adeno-associated virus vector for orthopaedic gene therapy. Clin Orthop Relat Res. 2000; (379 Suppl):S31-9. [DOI:10.1097/00003086-200010001-00005] [PMID]
- Fulton MD, Najahi-Missaoui W. Liposomes in cancer therapy: How did we start and where are we now. Int J Mol Sci. 2023; 24(7):6615. [DOI:10.3390/ijms24076615] [PMID] [PMCID]
- Du X, Zhao M, Jiang L, Pang L, Wang J, Lv Y, et al. A mini-review on gene delivery technique using nanoparticles-mediated photoporation induced by nanosecond pulsed laser. Drug Deliv. 2024; 31(1):2306231. [DOI:10.1080/10717544.2024.2306231] [PMID] [PMCID]
- Belhadj Z, Qie Y, Carney RP, Li Y, Nie G. Current advances in non-viral gene delivery systems: Liposomes versus extracellular vesicles. BMEMat. 2023; 1(2):e12018. [DOI:10.1002/bmm2.12018]
- Abdallah ABE, El-Ghannam MA, Hasan AA, Mohammad LG, Mesalam NM, Alsayed RM. Selenium nanoparticles modulate steroidogenesis-related genes and improve ovarian functions via regulating androgen receptors expression in polycystic ovary syndrome rat model. Biol Trace Elem Res. 2023; 201(12):5721-33. [DOI:10.1007/s12011-023-03616-0] [PMID] [PMCID]
- Mankin HJ. The response of articular cartilage to mechanical injury. J Bone Joint Surg Am. 1982; 64(3):460-6. [DOI:10.2106/00004623-198264030-00022] [PMID]
- Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994; 331(14):889-95. [DOI:10.1056/NEJM199410063311401] [PMID]
- Buckwalter JA, Mankin HJ. Articular cartilage repair and transplantation. Arthritis Rheum. 1998; 41(8):1331-42. [DOI:10.1002/1529-0131(199808)41:83.0.CO;2-J] [PMID]
- Mason JM, Grande DA, Barcia M, Grant R, Pergolizzi RG, Breitbart AS. Expression of human bone morphogenic protein 7 in primary rabbit periosteal cells: Potential utility in gene therapy for osteochondral repair. Gene Ther. 1998; 5(8):1098-104. [DOI:10.1038/sj.gt.3300703] [PMID]
- Smith P, Shuler FD, Georgescu HI, Ghivizzani SC, Johnstone B, Niyibizi C, et al. Genetic enhancement of matrix synthesis by articular chondrocytes: Comparison of different growth factor genes in the presence and absence of interleukin-1. Arthritis Rheum. 2000;43(5):1156-64. [DOI:10.1002/1529-0131(200005)43:53.0.CO;2-M] [PMID]
- Grande DA, Mason J, Light E, Dines D. Stem cells as platforms for delivery of genes to enhance cartilage repair. J Bone Joint Surg Am. 2003; 85-A Suppl 2:111-6. [DOI:10.2106/00004623-200300002-00015] [PMID]
- Lind M, Bünger C. Orthopaedic applications of gene therapy. Int Orthop. 2005; 29(4):205-9. [DOI:10.1007/s00264-005-0650-x] [PMID] [PMCID]
- Arnoczky SP, Warren RF. The microvasculature of the meniscus and its response to injury. An experimental study in the dog. Am J Sports Med. 1983; 11(3):131-41. [DOI:10.1177/036354658301100305] [PMID]
- Goto H, Shuler FD, Niyibizi C, Fu FH, Robbins PD, Evans CH. Gene therapy for meniscal injury: enhanced synthesis of proteoglycan and collagen by meniscal cells transduced with a TGFbeta(1)gene. Osteoarthritis Cartilage. 2000; 8(4):266-71. [DOI:10.1053/joca.1999.0300] [PMID]
- Csintalan RP, Inacio MC, Funahashi TT. Incidence rate of anterior cruciate ligament reconstructions. Perm J. 2008; 12(3):17-21. [DOI:10.7812/TPP/07-140] [PMID] [PMCID]
- Nakamura N, Shino K, Natsuume T, Horibe S, Matsumoto N, Kaneda Y, et al. Early biological effect of in vivo gene transfer of platelet-derived growth factor (PDGF)-B into healing patellar ligament. Gene Ther. 1998; 5(9):1165-70. [DOI:10.1038/sj.gt.3300712] [PMID]
- Lou J, Kubota H, Hotokezaka S, Ludwig FJ, Manske PR. In vivo gene transfer and overexpression of focal adhesion kinase (pp125 FAK) mediated by recombinant adenovirus-induced tendon adhesion formation and epitenon cell change. J Orthop Res. 1997; 15(6):911-8. [DOI:10.1002/jor.1100150618] [PMID]
- Martinek V, Latterman C, Usas A, Abramowitch S, Woo SL, Fu FH, et al. Enhancement of tendon-bone integration of anterior cruciate ligament grafts with bone morphogenetic protein-2 gene transfer: A histological and biomechanical study. J Bone Joint Surg Am. 2002; 84(7):1123-31. [DOI:10.2106/00004623-200207000-00005] [PMID]
- Baniasadi M, Talebi S, Mokhtari K, Zabolian AH, Khosroshahi EM, Entezari M, et al. Role of non-coding RNAs in osteoporosis. Pathology-Research and Practice. 2023; 155036. [DOI:10.1016/j.prp.2023.155036] [PMID]
- Turgeman G, Pittman DD, Müller R, Kurkalli BG, Zhou S, Pelled G, et al. Engineered human mesenchymal stem cells: A novel platform for skeletal cell mediated gene therapy. J Gene Med. 2001; 3(3):240-51. [DOI:10.1002/1521-2254(200105/06)3:33.0.CO;2-A] [PMID]
- Kostenuik PJ, Bolon B, Morony S, Daris M, Geng Z, Carter C, et al. Gene therapy with human recombinant osteoprotegerin reverses established osteopenia in ovariectomized mice. Bone. 2004; 34(4):656-64. [DOI:10.1016/j.bone.2003.12.006] [PMID]
- Akbar MA, Cao JJ, Lu Y, Nardo D, Chen MJ, Elshikha AS, et al. Alpha-1 antitrypsin gene therapy ameliorates bone loss in ovariectomy-induced osteoporosis mouse model. Hum Gene Ther. 2016; 27(9):679-86. [DOI:10.1089/hum.2016.029] [PMID]
- Abboud SL, Woodruff K, Liu C, Shen V, Ghosh-Choudhury N. Rescue of the osteopetrotic defect in op/op mice by osteoblast-specific targeting of soluble colony-stimulating factor-1. Endocrinology. 2002; 143(5):1942-9. [DOI:10.1210/endo.143.5.8775] [PMID]
- Glorieux FH, Rowe D. Osteogenesis imperfecta. Pediatr Bone. 2012; 511-39. [DOI:10.1016/B978-0-12-382040-2.10019-X]
- Niyibizi C, Smith P, Mi Z, Phillips CL, Robbins P. Transfer of proalpha2(I) cDNA into cells of a murine model of human Osteogenesis Imperfecta restores synthesis of type I collagen comprised of alpha1(I) and alpha2(I) heterotrimers in vitro and in vivo. J Cell Biochem. 2001; 83(1):84-91. [DOI:10.1002/jcb.1209] [PMID]
- Fernandes J, Tardif G, Martel-Pelletier J, Lascau-Coman V, Dupuis M, Moldovan F, et al. In vivo transfer of interleukin-1 receptor antagonist gene in osteoarthritic rabbit knee joints: Prevention of osteoarthritis progression. Am J Pathol. 1999; 154(4):1159-69. [DOI:10.1016/S0002-9440(10)65368-0] [PMID]
- Altman DA, Titus L, Hair GA, Boden SD. Molecular biology and spinal disorders. A survey for the clinician. Spine. 1999; 24(7):723-30. [DOI:10.1097/00007632-199904010-00023] [PMID]
- Cook SD, Dalton JE, Tan EH, Whitecloud TS 3rd, Rueger DC. In vivo evaluation of recombinant human osteogenic protein (rhOP-1) implants as a bone graft substitute for spinal fusions. Spine. 1994; 19(15):1655-63. [DOI:10.1097/00007632-199408000-00002] [PMID]
- MA M. The marshall urist young investigator award. gene expression during autograft lumbar spine fusion and the effect of bone morphogenetic protein 2. Clin Orthop Relat Res. 1998; 351:252-65. [Link]
- Boden SD, Titus L, Hair G, Liu Y, Viggeswarapu M, Nanes MS, et al. Lumbar spine fusion by local gene therapy with a cDNA encoding a novel osteoinductive protein (LMP-1). Spine. 1998; 23(23):2486-92. [DOI:10.1097/00007632-199812010-00003] [PMID]
- Kim HS, Viggeswarapu M, Boden SD, Liu Y, Hair GA, Louis-Ugbo J, et al. Overcoming the immune response to permit ex vivo gene therapy for spine fusion with human type 5 adenoviral delivery of the LIM mineralization protein-1 cDNA. Spine. 2003; 28(3):219-26. [DOI:10.1097/00007632-200302010-00004] [PMID]
- Helm GA, Alden TD, Beres EJ, Hudson SB, Das S, Engh JA, et al. Use of bone morphogenetic protein-9 gene therapy to induce spinal arthrodesis in the rodent. J Neurosurg. 2000; 92(2 Suppl):191-6. [DOI:10.3171/spi.2000.92.2.0191] [PMID]
- Adams MA, Roughley PJ. What is intervertebral disc degeneration, and what causes it? Spine. 2006; 31(18):2151-61. [DOI:10.1097/01.brs.0000231761.73859.2c] [PMID]
- Oichi T, Taniguchi Y, Oshima Y, Tanaka S, Saito T. Pathomechanism of intervertebral disc degeneration. JOR spine. 2020;3(1):e1076. [DOI:10.1002/jsp2.1076] [PMID] [PMCID]
- Nishida K, Kang JD, Gilbertson LG, Moon SH, Suh JK, Vogt MT, et al. Modulation of the biologic activity of the rabbit intervertebral disc by gene therapy: An in vivo study of adenovirus-mediated transfer of the human transforming growth factor beta 1 encoding gene. Spine. 1999; 24(23):2419-25. [DOI:10.1097/00007632-199912010-00002] [PMID]
- Wehling P, Cleveland SJ, Heininger K, Schulitz KP, Reinecke J, Evans CH. Neurophysiologic changes in lumbar nerve root inflammation in the rat after treatment with cytokine inhibitors. Evidence for a role of interleukin-1. Spine. 1996; 21(8):931-5. [DOI:10.1097/00007632-199604150-00005] [PMID]
- Remedios A. Bone and bone healing. Vet Clin North Am Small Anim Pract. 1999; 29(5):1029-44. [DOI:10.1016/S0195-5616(99)50101-0] [PMID]
- Buza JA 3rd, Einhorn T. Bone healing in 2016. Clin Cases Miner Bone Metab. 2016; 13(2):101-05. [DOI:10.11138/ccmbm/2016.13.2.101] [PMID] [PMCID]
- Rodríguez-Merchán EC. A review of recent developments in the molecular mechanisms of bone healing. Int J Mol Sci. 2021; 22(2):767. [DOI:10.3390/ijms22020767] [PMID] [PMCID]
- Lieberman JR, Daluiski A, Stevenson S, Wu L, McAllister P, Lee YP, et al. The effect of regional gene therapy with bone morphogenetic protein-2-producing bone-marrow cells on the repair of segmental femoral defects in rats. J Bone Joint Surg Am. 1999; 81(7):905-17. [DOI:10.2106/00004623-199907000-00002] [PMID]
- Rosen V. BMP and BMP inhibitors in bone. Ann N Y Acad Sci. 2006; 1068:19-25. [DOI:10.1196/annals.1346.005] [PMID]
- Bahamonde ME, Lyons KM. BMP3: To be or not to be a BMP. JBJS. 2001; 83(1):S56-62. [DOI:10.2106/00004623-200100001-00008]
- Fang J, Zhu YY, Smiley E, Bonadio J, Rouleau JP, Goldstein SA, et al. Stimulation of new bone formation by direct transfer of osteogenic plasmid genes. Proc Natl Acad Sci U S A. 1996; 93(12):5753-8. [DOI:10.1073/pnas.93.12.5753] [PMID] [PMCID]
- Bailón-Plaza A, Lee AO, Veson EC, Farnum CE, van der Meulen MC. BMP-5 deficiency alters chondrocytic activity in the mouse proximal tibial growth plate. Bone. 1999; 24(3):211-6. [DOI:10.1016/S8756-3282(98)00171-9] [PMID]
- Vukicevic S, Grgurevic L. BMP-6 and mesenchymal stem cell differentiation. Cytokine Growth Factor Rev. 2009; 20(5-6):441-8. [DOI:10.1016/j.cytogfr.2009.10.020] [PMID]
- Bertone AL, Pittman DD, Bouxsein ML, Li J, Clancy B, Seeherman HJ. Adenoviral-mediated transfer of human BMP-6 gene accelerates healing in a rabbit ulnar osteotomy model. J Orthop Res. 2004; 22(6):1261-70. [DOI:10.1016/j.orthres.2004.03.014] [PMID]
- Stojanović S, Tijanić M, Jovanović G, Spasić M, Stojković B, Petrović MB, et al. The role of growth factors in extraction wound healing. Acta stomatologica Naissi. 2015; 31(72):1524-37. [DOI:10.5937/asn1572524S]
- Meenan RF, Gertman PM, Mason JH. Measuring health status in arthritis. The arthritis impact measurement scales. Arthritis Rheum. 1980; 23(2):146-52. [DOI:10.1002/art.1780230203] [PMID]
- American College of Rheumatology Subcommittee on Rheumatoid Arthritis Guidelines. Guidelines for the management of rheumatoid arthritis: 2002 Update. Arthritis Rheum. 2002; 46(2):328-46. [DOI:10.1002/art.10148] [PMID]
- Lim DS, Kang MS, Jeong JA, Bae YS. Semi-mature DC are immunogenic and not tolerogenic when inoculated at a high dose in collagen-induced arthritis mice. Eur J Immunol. 2009; 39(5):1334-43. [DOI:10.1002/eji.200838987] [PMID]
- Iwaszko M, Biały S, Bogunia-Kubik K. Significance of interleukin (IL)-4 and IL-13 in inflammatory arthritis. Cells. 2021; 10(11):3000. [DOI:10.3390/cells10113000] [PMID] [PMCID]
- Woods JM, Katschke KJ Jr, Tokuhira M, Kurata H, Arai KI, Campbell PL, et al. Reduction of inflammatory cytokines and prostaglandin E2 by IL-13 gene therapy in rheumatoid arthritis synovium. J Immunol. 2000; 165(5):2755-63. [DOI:10.4049/jimmunol.165.5.2755] [PMID]
- Jacques C, Gosset M, Berenbaum F, Gabay C. The role of IL-1 and IL-1Ra in joint inflammation and cartilage degradation. Vitam Horm. 2006; 74:371-403. [DOI:10.1016/S0083-6729(06)74016-X] [PMID]
- Cutolo M. [IL-1Ra: its role in rheumatoid arthritis (Italian)]. Reumatismo. 2004; 56(Suppl 1):41-5. [DOI:10.4081/reumatismo.2004.1s.41] [PMID]
- Vermeij EA, Broeren MG, Bennink MB, Arntz OJ, Gjertsson I, van Lent PL, et al. Disease-regulated local IL-10 gene therapy diminishes synovitis and cartilage proteoglycan depletion in experimental arthritis. Ann Rheum Dis. 2015; 74(11):2084-91. [DOI:10.1136/annrheumdis-2014-205223] [PMID]
- Boyle DL, Nguyen KH, Zhuang S, Shi Y, McCormack JE, Chada S, Firestein GS. Intra-articular IL-4 gene therapy in arthritis: Anti-inflammatory effect and enhanced th2activity. Gene Ther. 1999; 6(12):1911-8. [DOI:10.1038/sj.gt.3301049] [PMID]
- Kobayashi T, Okamoto K, Kobata T, Hasunuma T, Kato T, Hamada H, et al. Novel gene therapy for rheumatoid arthritis by FADD gene transfer: Induction of apoptosis of rheumatoid synoviocytes but not chondrocytes. Gene Ther. 2000; 7(6):527-33. [DOI:10.1038/sj.gt.3301127] [PMID]
- Hildebrand KA, Gallant-Behm CL, Kydd AS, Hart DA. The basics of soft tissue healing and general factors that influence such healing. Sports Med Arthrosc Rev. 2005; 13(3):136-44. [DOI:10.1097/01.jsa.0000173230.61276.f4]
- Khanna A, Nelmes RT, Gougoulias N, Maffulli N, Gray J. The effects of LIPUS on soft-tissue healing: A review of literature. Br Med Bull. 2009; 89:169-82. [DOI:10.1093/bmb/ldn040] [PMID]
- Schwarz F, Jennewein M, Bubel M, Holstein JH, Pohlemann T, Oberringer M. Soft tissue fibroblasts from well healing and chronic human wounds show different rates of myofibroblasts in vitro. Mol Biol Rep. 2013; 40(2):1721-33. [DOI:10.1007/s11033-012-2223-6] [PMID]
- Braddock M, Campbell CJ, Zuder D. Current therapies for wound healing: Electrical stimulation, biological therapeutics, and the potential for gene therapy. Int J Dermatol. 1999; 38(11):808-17. [DOI:10.1046/j.1365-4362.1999.00832.x] [PMID]
- Chandler LA, Doukas J, Gonzalez AM, Hoganson DK, Gu DL, Ma C, et al. FGF2-Targeted adenovirus encoding platelet-derived growth factor-B enhances de novo tissue formation. Mol Ther. 2000; 2(2):153-60. [DOI:10.1006/mthe.2000.0102] [PMID]
- Helm GA, Li JZ, Alden TD, Hudson SB, Beres EJ, Cunningham M, et al. A light and electron microscopic study of ectopic tendon and ligament formation induced by bone morphogenetic protein-13 adenoviral gene therapy. J Neurosurg. 2001; 95(2):298-307. [DOI:10.3171/jns.2001.95.2.0298] [PMID]
- Ma Y, Zhang X, Wang J, Liu P, Zhao L, Zhou C, et al. Effect of bone morphogenetic protein-12 gene transfer on posterior cruciate ligament healing in a rabbit model. Am J Sports Med. 2009; 37(3):599-609. [DOI:10.1177/0363546508325960] [PMID]
- Ritter J, Bielack SS. Osteosarcoma. Ann Oncol. 2010; 21 (Suppl 7):vii320-5. [DOI:10.1093/annonc/mdq276] [PMID]
- Gorlick R, Khanna C. Osteosarcoma. J Bone Miner Res. 2010; 25(4):683-91. [DOI:10.1002/jbmr.77] [PMID]
- Jin P, Zhao Y, Liu H, Chen J, Ren J, Jin J, et al. Interferon-γ and tumor necrosis factor-α polarize bone marrow stromal cells uniformly to a Th1 phenotype. Sci Rep. 2016; 6:26345. [DOI:10.1038/srep26345] [PMID] [PMCID]
- Hughes DA. Gaucher disease. Washington: GeneReviews; 2023. [Link]
- Nagral A. Gaucher disease. J Clin Exp Hepatol. 2014; 4(1):37-50. [DOI:10.1016/j.jceh.2014.02.005] [PMID] [PMCID]
- Kim EY, Hong YB, Lai Z, Kim HJ, Cho YH, Brady RO, et al. Expression and secretion of human glucocerebrosidase mediated by recombinant lentivirus vectors in vitro and in vivo: Implications for gene therapy of Gaucher disease. Biochem Biophys Res Commun. 2004; 318(2):381-90. [DOI:10.1016/j.bbrc.2004.04.040]
- Figueiredo L, Souza L, Orellana M, Covas D, Lichtenstein F, Siciliano V, et al. A novel synthetic and recombinant glucocerebrosidase for gaucher disease: In silico molecular evolution and gene therapy approaches to enhance enzyme activity. Hematol Transfus Cell Ther. 2023; 45(4):S839. [DOI:10.1016/j.htct.2023.09.1512]
- Dunbar CE, Kohn DB, Schiffmann R, Barton NW, Nolta JA, Esplin JA, et al. Retroviral transfer of the glucocerebrosidase gene into CD34+ cells from patients with Gaucher disease: In vivo detection of transduced cells without myeloablation. Hum Gene Ther. 1998; 9(17):2629-40. [DOI:10.1089/hum.1998.9.17-2629] [PMID]
- Haase G, Kennel P, Pettmann B, Vigne E, Akli S, Revah F, et al. Gene therapy of murine motor neuron disease using adenoviral vectors for neurotrophic factors. Nat Med. 1997; 3(4):429-36. [DOI:10.1038/nm0497-429] [PMID]
- Pereira Lopes FR, Lisboa BC, Frattini F, Almeida FM, Tomaz MA, et al. Enhancement of sciatic nerve regeneration after vascular endothelial growth factor (VEGF) gene therapy. Neuropathol Appl Neurobiol. 2011; 37(6):600-12. [DOI:10.1111/j.1365-2990.2011.01159.x] [PMID]
- Gendron NH, MacKenzie AE. Spinal muscular atrophy: Molecular pathophysiology. Curr Opin Neurol. 1999; 12(2):137-42. [DOI:10.1097/00019052-199904000-00002] [PMID]
- Gartsman GM, Khan M, Hammerman SM. Arthroscopic repair of full-thickness tears of the rotator cuff. J Bone Joint Surg Am. 1998; 80(6):832-40. [DOI:10.2106/00004623-199806000-00007] [PMID]
- Harryman 2nd D, Mack L, Wang K, Jackins S, Richardson M, Matsen 3rd F. Repairs of the rotator cuff. Correl Funct Res Integr Cuff. 1991; 73(7):982-9. [DOI:10.2106/00004623-199173070-00004]
- Rokito AS, Cuomo F, Gallagher MA, Zuckerman JD. Long-term functional outcome of repair of large and massive chronic tears of the rotator cuff. J Bone Joint Surg Am. 1999; 81(7):991-7. [DOI:10.2106/00004623-199907000-00012] [PMID]
- Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004; 86(2):219-24. [DOI:10.2106/00004623-200402000-00002] [PMID]
- Gerber C, Fuchs B, Hodler J. The results of repair of massive tears of the rotator cuff. J Bone Joint Surg Am. 2000; 82(4):505-15. [DOI:10.2106/00004623-200004000-00006] [PMID]
- Postel JM, Goutallier D, Lavau L, Bernageau J. Anatomical results of rotator cuff repairs: Study of 57 cases controlled by arthrography. J Shoulder Elbow Surg. 1994; 3(20):320. [Link]
- Rodeo S, Potter H, Turner S, Campbel D, Kim H, Atkinson B. Augmentation of rotator cuff tendon healing using an osteoinductive growth factor. Orthopaedic Research Society Annual Meeting. 13 February 2002; Dallas, TX, US. [Link]
- Uggen JC, Dines J, Uggen CW, Mason JS, Razzano P, Dines D, et al. Tendon gene therapy modulates the local repair environment in the shoulder. J Osteopath Med. 2005; 105(1):20-1. [DOI:10.7556/jaoa.2005.105.1.20]
- Huard J, Fu FH. Gene therapy and tissue engineering in orthopaedic and sports medicine. Berlin: Springer Science & Business Media; 2000. [DOI:10.1007/978-1-4612-2126-5]
- Evans CH, Bandara G, Robbins PD, Mueller GM, Georgescu HI, Glorioso JC. Gene therapy for ligament healing. In: Jackson DW, editor. The anterior cruciate ligament: Current and future concepts. British Columbia: Raven Press; 1993. [Link]
- Woo S. Anatomy, biology, and biomechanics of tendon and ligament. In: Einhorn TA, Buckwalter JA, Simon SR, editors. Orthopaedic basic science: Biology and blamechanics of the musculoskeletal system. Illinois: American Academy of Orthopaedic Surgeons; 2000. [Link]
- Nakase T, Sugamoto K, Miyamoto T, Tsumaki N, Luyten FP, Inui H, et al. Activation of cartilage-derived morphogenetic protein-1 in torn rotator cuff. Clin Orthop Relat Res. 2002; (399):140-5. [DOI:10.1097/00003086-200206000-00016] [PMID]
- Yoshikawa Y, Abrahamsson SO. Dose-related cellular effects of platelet-derived growth factor-BB differ in various types of rabbit tendons in vitro. Acta Orthop Scand. 2001; 72(3):287-92. [DOI:10.1080/00016470152846646] [PMID]
- Dahlgren LA, van der Meulen MC, Bertram JE, Starrak GS, Nixon AJ. Insulin-like growth factor-I improves cellular and molecular aspects of healing in a collagenase-induced model of flexor tendinitis. J Orthop Res. 2002; 20(5):910-9. [DOI:10.1016/S0736-0266(02)00009-8] [PMID]
- Dahlgren LA, Nixon AJ, Brower-Toland BD. Effects of beta-aminopropionitrile on equine tendon metabolism in vitro and on effects of insulin-like growth factor-I on matrix production by equine tenocytes. Am J Vet Res. 2001; 62(10):1557-62. [DOI:10.2460/ajvr.2001.62.1557] [PMID]
Type of Study: Review Paper |
Subject:
Shoulder / Elbow
Received: 2022/05/1 | Accepted: 2022/06/19 | Published: 2023/05/1
Received: 2022/05/1 | Accepted: 2022/06/19 | Published: 2023/05/1
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |






